Abstract
Circulating alloantibodies are found in a substantial number of renal allograft recipients, and can induce chronic allograft injury, which is represented microscopically as transplant glomerulopathy and diffuse C4d deposition in peritubular capillaries (PTCs). Development of these injuries is significantly correlated with late allograft loss, and in this regard, it was included as a new disease entity named chronic antibody-mediated rejection (cAMR) in the updated Banff 05 classification. Usually, the prognosis of cAMR is poor and conventional immunosuppressants mainly targeting T cell-mediated immunity cannot prevent or reverse it. Therefore, some researchers have suggested that therapies directed at the humoral response may be required for the treatment of cAMR. Recently, some reports have suggested that the combined use of rituximab and intravenous immunoglobulin (IVIg) therapy may be useful for the treatment of cAMR. Our previous study also showed that rituximab and IVIg combination therapy effectively delayed the progression of cAMR. We administered rituximab and IVIg combination therapy to 18 biopsy-proven cAMR patients and found that it significantly slowed the decline of the estimated glomerular filtration rate. However, this effect was limited in patients with heavy proteinuria, and dissipated in all patients by 1 year post-treatment. Recently, new drugs targeting the humoral immune system, such as bortezomib and eculizumab, have been tested for the treatment of cAMR. However, the studies still lack definitive data in terms of successful treatment of cAMR. We speculate that those therapies will compensate for the limitation of previous anti-humoral therapies for cAMR.
Chronic antibody-mediated rejection (cAMR), one of the main causes of late allograft loss, was suggested as a new disease entity for the first time in 2001 [1]. This study showed that a significant proportion of chronic rejection cases are mediated by alloantibodies, and C4d positivity can separate these cases from non-specific chronic allograft nephropathy [2]. Another study reported that complement activation in renal microvasculature may result in C4d deposition, characterized by typical features such as chronic transplant arteriopathy, glomerulopathy, and basement multilayering in peritubular capillaries (PTCs) [3]. Since then, several studies have suggested that alloantibody-induced chronic renal allograft injury should be distinguished from chronic T cell-mediated rejection [4,5,6]. Therefore, the updated Banff 2005 classification added cAMR as a category of antibody-mediated rejection (AMR) [4,7,8]. Recently, this disease--initially identified as non-specific chronic rejection--has received increased attention as a major contributor of graft failure cases, and conventional immunosuppressants have been rendered unsuitable to prevent or reverse cAMR [2,9]. In this review, we introduce the clinical significance of cAMR and discuss the current issues in the treatment of this disease.
The detailed mechanism for the development of cAMR has not been fully investigated; however, some studies suggest that antibody-mediated injury is the main pathogenic mechanism [10,11,12]. For example, biopsy-confirmed chronic rejection was preceded by the detection of de novo alloantibodies in the majority of cases [13]. In addition, circulating alloantibodies are found in a substantial number of renal allograft recipients with long-term follow up, and significantly correlated with the development of late graft loss [10,14,15]. Histologically, chronic allograft injuries due to alloantibodies manifest as transplant glomerulopathy, peritubular capillary basement membrane multilayering on electron microscope, and antibody interaction with vascular endothelium was shown as diffuse C4d deposition in PTCs, or microvascular inflammation (g + ptc score) [5,9,16,17].
Based on the evidence provided above, and the updated Banff classification, the diagnosis of cAMR is based on the following findings: (1) transplant glomerulopathy and severe PTC basement mem-brane multilayering, interstitial fibrosis, and tubular atrophy with or without PTC loss, along with fibrous intimal thickening in arteries without internal elastica duplication; and (2) diffuse C4d deposition in PTCs or moderate microvascular inflammation (g + ptc≥2) or increased expression of gene transcript indicative of endothelial injury; and (3) presence of anti-human leukocyte antigen donor-specific antibodies (HLA-DSA) [7,17,18].
As mentioned above, development of cAMR is mediated by the activation of the humoral immune system; hence, the presence of HLA-DSA may be significantly associated with the development of cAMR. Indeed, the incidence of cAMR in patients with a positive crossmatch before kidney transplantation (KT) was 22% at 1-year posttransplantation. However, in ABO incompatible KT or conventional KT with low immunologic risk, the incidence was only 13% and 8%, respectively [19]. In another report, the rate of cAMR was nearly 50% in patients with a low titer of HLA-DSA who were not desensitized before KT. On the other hand, desensitization using rituximab and plasmapheresis effectively prevented the development of cAMR [20,21].
In different to acute antibody mediated rejection, which is characterized by abrupt deterioration of allograft function, cAMR show-ed indolent course characterized by gradual decline of allograft function over the years [22]. The typical clinical course of cAMR is presented in Fig. 1. The initial step is accommodation, in which circulating alloantibodies do not attack the allograft tissue. This is followed by subclinical AMR, in which allograft tissue injury progresses but its function is not compromised, and finally, overt cAMR with graft dysfunction [23]. Considering the clinical course, cAMR is usually detected 1 year after transplantation, and is characterized by a slow progressive loss of graft function, accompanied by proteinuria of various ranges [1,22,24]. Majority of studies suggest that the prognosis of cAMR is unfavorable [9,25]. For example, when transplant glomerulopathy, which is the hallmark of cAMR, is detected using protocol biopsies, nearly 50% of patients return to dialysis within 5 years. However, due to the heterogeneity of histologic findings in cAMR, no convincing data exist regarding the outcome of cAMR, with a diagnosis based on the Banff criteria. A well-designed prospective trial would be required to determine this issue.
As mentioned above, the mechanism of cAMR has not been fully elucidated; hence, an established treatment guideline does not exist [2,7,8]. The use of tacrolimus and mycophenolate mofetil rescue therapy in cAMR does not show significant improvement in allograft function, which means that a conventional immunosuppressant regimen cannot prevent or reverse it [3]. Some resear-chers have proposed that therapies directed at the humoral immune response may be required to successfully treat cAMR, since antibody-mediated tissue injury, rather than T-cell mediated immunity, is associated with its development [21,22]. Thus far, several drugs have been introduced to suppress the various steps of humoral immune reactions, and several studies that apply these drugs for the treatment of cAMR are on-going (Fig. 2) [12,23,26].
Rituximab is a chimeric anti-CD20 monoclonal antibody that can induce antibody-dependent cell-mediated cytotoxicity, complement-dependent cell killing, and induction of apoptotic cell death, especially in B cells [27,28]. Initially, it was used in hematology for the treatment of malignant lymphoma or leukemia, but it was introduced in kidney transplantation because of its suppressive effect on humoral immunity [27]. Indeed, the use of rituximab in the introductory period effectively reduces the rate of cAMR after KT. For example, in ABO compatible KT, the rate of cAMR was 28.9% 2 years after KT, but it only 3.5% in patients undergoing KT from an ABO incompatible donor with a desensitization protocol that included rituximab [29]. In another study that suggests the therapeutic effect of rituximab on cAMR, the allograft survival rate after the diagnosis of cAMR was superior in the rituximab treatment group than in the control group [30]. All the above findings suggest that rituximab could be a relevant treatment option for cAMR.
Intravenous immunoglobulin (IVIg) is known to have powerful and multiple immunomodulatory effects [31,32]. The exact mechanism of IVIg has not been elucidated, but it can suppress immunoglobulin synthesis, has anti-idiotypic activity against DSA with resultant neutralization of HLA-DSA, blocks the Fc receptor, inhibits complement activation, and has anti-cytokine activity [32]. In KT, a high dose of IVIg (2 g/kg) administered to highly sensitized patients significantly reduces the frequency of allosensitization and acute rejection episodes, resulting in improved long-term outcomes [33,34]. However, in spite of the proven efficacy of IVIg, its use is not completely effective in some patients, and the effect is not predictable in most cases. Hence, combination therapy with rituximab, rather than IVIg alone, has been evaluated for the treatment of cAMR as described below.
In recent years, rituximab and IVIg combination therapy (RIT) has been tested in patients with cAMR. Improved allograft function was observed after treatment, and hence, it is now accepted as the only treatment option with reported benefits [28,35,36]. At first, RIT was tested in 6 pediatric renal transplant recipients. They received 4 weekly doses of IVIg (1 g/[kg·dose]), followed by a single dose of rituximab (375 mg/m2 body surface area) 1 week after the last IVIg infusion. Following treatment, allograft function improv-ed or stabilized in 4 out of 6 recipients [28]. In a prospective study with a 2-year follow-up, this therapy significantly reduced or stabilized the progressive loss of transplant function in pediatric patients [35]. In adult patients with cAMR, RIT also showed similar effects in the improvement of allograft function in patients with cAMR [36].
Our previous reports also demonstrated that RIT effectively delayed cAMR progression. We administered rituximab (375 mg/m2) followed by IVIg (0.4 g/kg) for 4 days (Fig. 3). In our preliminary study with 6 patients, allograft function improved or was stabiliz-ed in 3 patients who showed relatively early stage cAMR [24]. In a larger study group (n=18) with longer follow-up duration, the response rate to RIT was 66.7% (12/18), with a significant decrease in the decline rate of estimated glomerular filtration rate (eGFR) in the 6 months after RIT, compared to the rate observed 6 months before RIT. Clinical and histological features between the 12 responders and the 6 non-responders were not significantly different. However, non-responders had significantly higher proteinuria levels during RIT, which suggests that the proteinuria level may be an important prognostic factor for the response to RIT [37].
Despite the proven effect of RIT in delaying the progression of cAMR, it showed some limitations. First, RIT was not completely effective in all cases of cAMR, and its effect was limited in patients with advanced allograft tissue injury or high-grade proteinuria [28,37]. This suggests that RIT may reverse the progression of earlier stage cAMR, but it cannot reverse advanced stage cAMR, which is characterized by advanced fibrotic changes in the allograft tissue. Second, in the long-term follow-up, the therapeutic effect of RIT showed a decreasing trend with time, especially at 1 year after RIT initiation. In our previous study, 4 patients with a follow-up duration >2 years were included, and a time-dependent decrease in eGFR was detected. Accordingly, repeated RIT therapy or other additional strategies for humoral immunity may be necessary to prolong the therapeutic effect [38,39,40]. Third, it is uncertain whether RIT promotes renal allograft survival, because the majority of previous studies were single-arm studies, which only investigate the change in clinical parameters that indicate allograft function before and after treatment. A randomized prospective trial may be required to prove its efficacy.
Bortezomib is a proteasome inhibitor that has a suppressive effect on antibody production by plasma cells, stimulates apoptosis of this cell type, and decreases the number of bone marrow-derived plasma cells [41]. Initially, it was approved for the treatment of multiple myeloma by the Food and Drug Administration and has now been introduced for use in KT and it is expected to show a stronger suppressive effect on humoral immunity than rituximab [42,43]. In an animal model, bortezomib effectively ameliorated glomerular, tubulointerstitial, and vascular changes of cAMR via the inhibition of antibody-producing cells [44]. In the clinical setting, very few patients have received bortezomib as a rescue treatment for cAMR, producing inconsistent results. Hence, the therapeutic effect of this drug has not yet been proven [45]. A randomized controlled trial to investigate the effects of bortezomib on cAMR (known as the BORJECT study) is now in progress, which can help determine the effectiveness of this drug (NCT01873157).
One promising agent for the treatment of cAMR is eculizumab. This agent inhibits the cleavage of C5 into C5a and C5b, thereby preventing the formation of the membrane attack complex [46]. Eculizumab has been approved for the treatment of paroxysmal nocturnal hemoglobinuria by the Food and Drug Administration; however, recent data also shows that eculizumab effectively suppresses humoral immune responses in KT. For example, post-transplant use of eculizumab significantly decreases the development of acute AMR as well as transplant glomerulopathy at 1 year following KT [12]. In regards to cAMR treatment, a prospective study is in progress and actively enrolling candidates [47].
Mesenchymal stem cells (MSCs) are of interest in transplantation owing to their potential immune-modulating effect [48]. This therapeutic potential is mediated by multiple mechanisms through the secretion of regulatory cytokines, activation of regulatory immune cells, and the capacity to increase cellular repair through the secretion of anti-apoptotic, anti-fibrotic, and pro-angiogenic factors [49]. The multiple functions of MSCs may lead to multifaceted strategies in various organs and diseases, including KT [50,51]. Indeed, in a trial that included 159 patients undergoing renal transplantation, the use of autologous MSCs, compared with anti-interleukin-2 receptor antibody induction therapy, resulted in lower incidence of acute rejection [52]. Additionally, a study involving KT recipients, evaluating the effect of MSCs in chronic allograft nephropathy, has been registered by the Uzhou Institute of China.
Development of cAMR is a substantial obstacle to long-term allograft outcome. In the past decades, various therapeutic options such as RIT have been investigated, but has showed many limitations. Recently, several new therapeutic approaches, which can effectively suppress humoral immunity, such as bortezomib and eculizumab, have emerged. Extensive studies and longer follow-up may be needed to determine if these apparent advances will improve the outcome of cAMR.
Figures and Tables
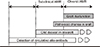 | Fig. 1Stages of chronic antibody mediated rejection. The first stage is accommodation, in which circulating alloantibodies do not attack allograft tissue. This is followed by subclinical AMR in which allograft tissue injury progresses but its function is not compromised. Finally, overt chronic antibody-mediated rejection with graft dysfunction develops and progresses to graft failure. AMR, antibody-mediated rejection. |
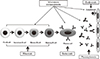 | Fig. 2Effect of drugs used for antibody-mediated rejection. Plasmapheresis removes circulating alloantibodies. Rituximab depletes B cells of all stages, and bortezomib directly suppresses antibody-producing plasma cells. Intravenous immunoglobulin can modulate various immune reactions through multiple mechanisms. Eculizumab inhibits the terminal complement pathway. |
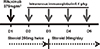 | Fig. 3Rituximab/intravenous immunoglobulin combination therapy protocol for chronic antibody-mediated rejection. On the first day, rituximab (375 mg/m2) was administered followed by intravenous immunoglobulin (0.4 g/kg) for 4 days. Concomitantly, 250 mg of methylprednisolone was administered twice a day, intravenously, for a period of 3 days, followed by oral prednisolone. |
References
1. Sellares J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012; 12:388–399.

2. Mauiyyedi S, Pelle PD, Saidman S, Collins AB, Pascual M, Tolkoff-Rubin NE, et al. Chronic humoral rejection: identification of antibody-mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol. 2001; 12:574–582.

3. Regele H, Bohmig GA, Habicht A, Gollowitzer D, Schillinger M, Rockenschaub S, et al. Capillary deposition of complement split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: a contribution of humoral immunity to chronic allograft rejection. J Am Soc Nephrol. 2002; 13:2371–2380.

4. Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003; 349:2326–2333.

5. Vongwiwatana A, Gourishankar S, Campbell PM, Solez K, Halloran PF. Peritubular capillary changes and C4d deposits are associated with transplant glomerulopathy but not IgA nephropathy. Am J Transplant. 2004; 4:124–129.

6. Ishii Y, Sawada T, Kubota K, Fuchinoue S, Teraoka S, Shimizu A. Injury and progressive loss of peritubular capillaries in the development of chronic allograft nephropathy. Kidney Int. 2005; 67:321–332.

7. Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, et al. Banff '05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy ('CAN'). Am J Transplant. 2007; 7:518–526.

8. Aita K, Yamaguchi Y, Shimizu T, Horita S, Furusawa M, Tanabe K, et al. Histological analysis of late renal allografts of antidonor antibody positive patients with C4d deposits in peritubular capillaries. Clin Transplant. 2004; 18:Suppl 11. 7–12.

9. Cosio FG, Gloor JM, Sethi S, Stegall MD. Transplant glomerulopathy. Am J Transplant. 2008; 8:492–496.

10. Pascual J, Perez-Saez MJ, Mir M, Crespo M. Chronic renal allograft injury: early detection, accurate diagnosis and management. Transplant Rev (Orlando). 2012; 26:280–290.

11. Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, Hidalgo LG, et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2009; 9:2520–2531.

12. Stegall MD, Chedid MF, Cornell LD. The role of complement in antibody-mediated rejection in kidney transplantation. Nat Rev Nephrol. 2012; 8:670–678.

13. Lee PC, Terasaki PI, Takemoto SK, Lee PH, Hung CJ, Chen YL, et al. All chronic rejection failures of kidney transplants were preceded by the development of HLA antibodies. Transplantation. 2002; 74:1192–1194.

14. Everly MJ, Rebellato LM, Haisch CE, Ozawa M, Parker K, Briley KP, et al. Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation. 2013; 95:410–417.

15. Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012; 12:1157–1167.

16. Husain S, Sis B. Advances in the understanding of transplant glomerulopathy. Am J Kidney Dis. 2013; 62:352–363.

17. Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014; 14:272–283.

18. Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008; 8:753–760.

19. Gloor JM, Cosio FG, Rea DJ, Wadei HM, Winters JL, Moore SB, et al. Histologic findings one year after positive crossmatch or ABO blood group incompatible living donor kidney transplantation. Am J Transplant. 2006; 6:1841–1847.

20. Ishida H, Furusawa M, Shimizu T, Nozaki T, Tanabe K. Influence of preoperative anti-HLA antibodies on short- and long-term graft survival in recipients with or without rituximab treatment. Transpl Int. 2014; 27:371–382.

21. Hirai T, Kohei N, Omoto K, Ishida H, Tanabe K. Significance of low-level DSA detected by solid-phase assay in association with acute and chronic antibody-mediated rejection. Transpl Int. 2012; 25:925–934.

22. Pascual J, Perez-Saez MJ, Mir M, Crespo M. Chronic renal allograft injury: early detection, accurate diagnosis and management. Transplant Rev (Orlando). 2012; 26:280–290.

23. Colvin RB. Antibody-mediated renal allograft rejection: diagnosis and pathogenesis. J Am Soc Nephrol. 2007; 18:1046–1056.

24. Hong YA, Kim HG, Choi SR, Sun IO, Park HS, Chung BH, et al. Effectiveness of rituximab and intravenous immunoglobulin therapy in renal transplant recipients with chronic active antibody-mediated rejection. Transplant Proc. 2012; 44:182–184.

25. Terasaki PI, Ozawa M. Predictive value of HLA antibodies and serum creatinine in chronic rejection: results of a 2-year prospective trial. Transplantation. 2005; 80:1194–1197.

26. Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai CH, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008; 359:242–251.

27. Salama AD, Pusey CD. Drug insight: rituximab in renal disease and transplantation. Nat Clin Pract Nephrol. 2006; 2:221–230.

28. Billing H, Rieger S, Ovens J, Susal C, Melk A, Waldherr R, et al. Successful treatment of chronic antibody-mediated rejection with IVIG and rituximab in pediatric renal transplant recipients. Transplantation. 2008; 86:1214–1221.

29. Kohei N, Hirai T, Omoto K, Ishida H, Tanabe K. Chronic antibody-mediated rejection is reduced by targeting B-cell immunity during an introductory period. Am J Transplant. 2012; 12:469–476.

30. Smith RN, Malik F, Goes N, Farris AB, Zorn E, Saidman S, et al. Partial therapeutic response to Rituximab for the treatment of chronic alloantibody mediated rejection of kidney allografts. Transpl Immunol. 2012; 27:107–113.

31. Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001; 345:747–755.

32. Jordan SC, Vo AA, Peng A, Toyoda M, Tyan D. Intravenous gammaglobulin (IVIG): a novel approach to improve transplant rates and outcomes in highly HLA-sensitized patients. Am J Transplant. 2006; 6:459–466.

33. Jordan SC, Quartel AW, Czer LS, Admon D, Chen G, Fishbein MC, et al. Posttransplant therapy using high-dose human immunoglobulin (intravenous gammaglobulin) to control acute humoral rejection in renal and cardiac allograft recipients and potential mechanism of action. Transplantation. 1998; 66:800–805.

34. Jordan SC, Vo A, Bunnapradist S, Toyoda M, Peng A, Puliyanda D, et al. Intravenous immune globulin treatment inhibits crossmatch positivity and allows for successful transplantation of incompatible organs in living-donor and cadaver recipients. Transplantation. 2003; 76:631–636.

35. Billing H, Rieger S, Susal C, Waldherr R, Opelz G, Wuhl E, et al. IVIG and rituximab for treatment of chronic antibody-mediated rejection: a prospective study in paediatric renal transplantation with a 2-year follow-up. Transpl Int. 2012; 25:1165–1173.

36. Fehr T, Rusi B, Fischer A, Hopfer H, Wuthrich RP, Gaspert A. Rituximab and intravenous immunoglobulin treatment of chronic antibody-mediated kidney allograft rejection. Transplantation. 2009; 87:1837–1841.

37. An GH, Yun J, Hong YA, Khvan M, Chung BH, Choi BS, et al. The Effect of Combination Therapy with Rituximab and Intravenous Immunoglobulin on the Progression of Chronic Antibody Mediated Rejection in Renal Transplant Recipients. J Immunol Res. 2014; 2014:828732.

38. Clatworthy MR. Targeting B cells and antibody in transplantation. Am J Transplant. 2011; 11:1359–1367.

39. Everly MJ. A summary of bortezomib use in transplantation across 29 centers. Clin Transpl. 2009; 323–337.
40. Schwaiger E, Regele H, Wahrmann M, Werzowa J, Haidbauer B, Schmidt A, et al. Bortezomib for the treatment of chronic antibody-mediated kidney allograft rejection: a case report. Clin Transpl. 2010; 391–396.
41. Perry DK, Burns JM, Pollinger HS, Amiot BP, Gloor JM, Gores GJ, et al. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant. 2009; 9:201–209.

42. Everly MJ. An update on antibody reduction and rejection reversal following bortezomib use: a report of 52 cases across 10 centers. Clin Transpl. 2010; 353–362.
43. Yang KS, Jeon H, Park Y, Jo IH, Kim JI, Moon IS, et al. Use of bortezomib as anti-humoral therapy in kidney transplantation. J Korean Med Sci. 2014; 29:648–651.

44. Vogelbacher R, Meister S, Guckel E, Starke C, Wittmann S, Stief A, et al. Bortezomib and sirolimus inhibit the chronic active antibody-mediated rejection in experimental renal transplantation in the rat. Nephrol Dial Transplant. 2010; 25:3764–3773.

45. Kim MG, Kim YJ, Kwon HY, Park HC, Koo TY, Jeong JC, et al. Outcomes of combination therapy for chronic antibody-mediated rejection in renal transplantation. Nephrology (Carlton). 2013; 18:820–826.

46. Larrea CF, Cofan F, Oppenheimer F, Campistol JM, Escolar G, Lozano M. Efficacy of eculizumab in the treatment of recurrent atypical hemolytic-uremic syndrome after renal transplantation. Transplantation. 2010; 89:903–904.

47. Eculizumab Therapy for Chronic Complement-Mediated Injury in Kidney Transplantation. cited 2014 Jul 10. Available from: http://clinicaltrialsgov/show/NCT01327573.
48. Picinich SC, Mishra PJ, Glod J, Banerjee D. The therapeutic potential of mesenchymal stem cells. Cell- & tissue-based therapy. Expert Opin Biol Ther. 2007; 7:965–973.
49. Hematti P. Role of mesenchymal stromal cells in solid organ transplantation. Transplant Rev (Orlando). 2008; 22:262–273.

50. Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007; 292:F1626–F1635.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download