Abstract
Despite the great potential of ABO-incompatible (ABOi) liver transplantation (LT) for expanding the donor pool, serious concern about poor outcomes in the recipients has been a major obstacle to its widespread. The use of ABOi living donors is an attractive solution for expanding the liver donor pool, and various novel strategies for desensitization of ABO incompatibility have yielded promising results. The 1st breakthrough was local graft infusion therapy introduced by the Keio and Kyoto group; a second, epochal advance was the advent of the anti-CD20 monoclonal antibody, rituximab. Since then, the risk of fulminant hepatic necrosis caused by full-blown antibody-mediated rejection (AMR) has almost disappeared, and survival outcomes of ABOi LT have increased markedly. In the Korean experience, ABOi LT accounts for 18% of all adult living donor liver transplantation, and 3-year graft and patient survival rates are 86.5 and 87.6%, respectively. ABOi living donor LT is thus having a major impact on the donor pool and the recent achievements permit us to promote a nationwide ABOi LT program. However, concern still remains about diffuse intrahepatic biliary stricture (DIHBS), which is an attenuated form of AMR. Ultimately, we need to identify risk factors and preventive measures for this.
The annual number of liver transplantation (LT) in Korea has continuously increased, mainly due to the explosive rise in the number of living donor liver transplants (LDLT) (Fig. 1A) [1]. However, LT cannot keep pace with the increase in the size of the waiting list in Korea despite the recent gradual boost in deceased donor numbers (Fig. 1B) [1]. Therefore, expanding the liver donor pool has always been a major challenge for Korean society. The potential for expanding the deceased donor pool in our country seems to be limited and expansion of the living donor pool is generally hampered by insuperable ethical and donor safety issues. Hence, the involvement of ABO-incompatible (ABOi) donors is an attractive solution for the organ shortage because it does not add to donor risk and is potentially very effective. However, widespread application of ABOi LT has been limited by serious concerns about recipient outcomes due to disappointing early experience.
ABOi organ transplantation has definitely undergone a paradigm shift since the introduction of the anti-CD20 monoclonal antibody, rituximab. Survival outcomes have become comparable to those of ABO-compatible transplantation due to various novel strategies for desensitization (DSZ). In this review, I present a brief history of recent achievements in ABOi LT, focussing on current trends in DSZ protocols, provide the clinical outcomes of the Korean experience in ABOi LDLT and suggest further steps for improving clinical outcomes.
After Starzl, et al. breached the ABO blood group barrier in LT, the liver was regarded as an immunologically privileged organ [2]. Hence, ABO-incompatibility was not considered a contraindication for LT in the initial period. However, clinical outcomes in real practice were very disappointing due to a high incidence of early graft loss, which was associated with antibody-mediated rejection (AMR) [3,4]. To avoid AMR, basic unselective DSZ protocols such as whole-body irradiation, routine steroid pulse therapy or maintenance of extremely high trough levels of calcineurin inhibitor were employed. However, these failed to have any positive impact, and the majority of patient deaths in the earlier period of ABOi LT were associated with AMR, or infection due to ineffective immunosuppressionor harmful over-immunosuppression. Around that time, the incidence of 1-month graft loss ranged from 40-60%, and 3-year patient survival ranged from 20 to 40% [3,4]. Hence, ABOi LT became unpopular and has been reserved for urgent LT, particularly for pediatric patients [5]. Despite this, many trials have been undertaken in Asian countries aimed at improving the survival of recipients of ABOi adult living donor liver transplants (ALDLT). For example, Tanabe et al. infused anti-inflammatory agents directly into the graft via the portal vein (PV), which yielded significantly improved outcomes [6]. At around the same period, the Kyoto group reported a modified and more successful method of infusion, via the hepatic artery (HA), [7]. Since the adoption of rituximab in ABOi LT by Usuda et al. [8], the incidence of AMR in ABOi ALDLT has been markedly reduced and recent outcomes are comparable to those of ABO-compatible (ABOc) LT [9,10,11,12]. In the era of rituximab prophylaxis, ABO incompatibility is no longer considered a contraindication for LT.
ABOi LT has experienced its ups and downs. Recently, it has become the standard treatment option in countries with an extreme shortage of deceased organs owing to the remarkable improvement insurvival outcomes. However, it seems too early to proclaim a secure immunological victory because of the intractable morbidity due to intrahepatic biliary stricture, which is an attenuated form of AMR and occurs exclusively in ABOi LT. In addition, we need to standardize the usage of rituximab. Therefore, attention in the coming era should be focussed on finding a solution to these problems.
Damage to ABOi liver grafts is initiated by preformed isoagglutinin (IA) and is potentiated by the proliferation of B-cells, which are activated by ABO antigens in the donor graft. The main targets are the endothelial cells that line the graft vasculature, and the epithelial cells that line the bile duct. The resulting bile duct strictures and vascular thromboses cause graft ischemia, severe cholestasis and, eventually, graft loss. The histology of AMR is characterized by hemorrhagic necrosis with diffuse intravascular coagulation (DIC) within the graft, exemplifying single organ DIC [4]. Haga et al. [13] reported that periportal edema and necrosis appear ed to be histological indications of the early phase of severe humo ral rejection. In that report, all grafts displaying periportal edema and necrosis developed massive parenchymal or biliary necrosis. To potentiate the histologic diagnosis of AMR, immunohistochemical staining with anti-C4d may be helpful. C4d binds covalently to tissues after activation of the complement system, and immunostaining has been widely used to demonstrate humoral immunoreactivity or AMR in other solid organ transplants [14,15,16]. Although it is not a pathognomic feature of AMR, detection of C4d has both diagnostic and prognostic value, and may be a hallmark of antibody-mediated rejection in liver biopsies [17].
There is no difference in immunosuppressive therapy between ABOi and ABOc LDLT except DSZ protocol. In ABOi LDLT, however, several different DSZ protocols have been used to try to prevent IA-mediated graft injury. These protocols have three main aims: 1) pre-transplant reduction of IA titer, 2) attenuation of local inflammation, 3) suppression of B-cell activity. The commonly used DSZ protocol in ABOi ALDLT is depicted in Fig. 2.
Total plasma exchange (TPE), the simplest method for reducing IA titer, is generally used in DSZ. It takes 3-4 hours per session and uses fresh frozen blood type AB plasma. In one session of TPE one can filter 1.5 plasma volumes. This approach is used for lowering elevated IA titers during the post-transplant period, as well as pre-transplant [18]. The disadvantages of TPE are hypersensitivity, citrate toxicity, hemodynamic stress and relatively low efficiency and selectivity. To overcome the drawbacks of TPE, a Japanese group has developed double-filtration plasmapheresis [19], which permits selective removal of the plasma fraction containing the immunoglobulins. It consists of two systems, plasma separator and fractionator. Removal of coagulation factors can be avoided and only small amounts of replacement fluid are needed. However, outside Japan the use of double-filtration plasmapheresis is very limited. More recently, a selective approach using antigen-specific immunoadsorption with immunoadsorbentcolumns (GlycoSorb-ABO; Glycorex Transplantation AB, Lund, Sweden) has been introduced [20]. It employs a conventional plasmapheresis device supporting selective GlycoSorb ABO immunoadsorbent columns selectively depleting anti-A and anti-B isoagglutinins. It was developed in order to minimize the side-effects and morbidity related to TPE. It has been widely used in ABOi kidney transplantation, but only in a few reported liver transplant recipients [21].
Based on an understanding of the pathophysiologic mechanism of AMR, i.e., local DIC, direct infusion of anti-inflammatory drugs such as prostaglandin E1, methylprednisolone, and gabexatemesylate through a portal vein (PV) or hepatic artery (HA) catheter, was an innovative method of improving survival outcomes in ABOi ALDLT in Japan. It was once regarded as an essential component of the DSZ protocol. However, the increasing use of rituximab to prevent AMR has led some to question the role of local graft infusion therapy (LGIT). This is because LGIT does not prevent AMR. In fact, the technique often causes complications such as bleeding and vascular thrombosis. Egawa et al. [10] reported LGIT-related complications in 16-37% of patients. The majority of LGIT-related complications in cases of PV infusion are due to PV thrombosis. Also, catheter dislocation often causes intra-abdominal bleeding. The complication rate associated with HA infusion is slightly lower; however, the complications associated with HA catheters, such as HA injury, thrombosis, and catheter dislocation, are potentially lethal. Although there is still some controversy about the clinical usefulness of LGIT, recent clinical studies have obtained success with DSZ protocols without LGIT [22,23].
To prevent a post-transplant rebound rise of IA, one needs to suppress B-cell activity by surgical or pharmacological intervention. For that reason, splenectomy (SPL) was once considered an essential component of successful DSZ. However, the impact of SPL upon B-cell suppression is not consistent and SPL is always accompanied by the danger of bleeding, portal vein thrombosis or pancreatic fistula, etc. In addition, it has been reported that rituximab can completely replace the role of SPL; the Kyoto group have reported that splenectomy does not provide any immunological benefit in ABOi LDLT [24].
Rituximab, a chimeric murine/human monoclonal antibody, reacts with the CD20 antigen. Its exact mechanism of action is still under a veil. Three different mechanisms have been proposed for how it eliminates B cells: complement dependent killing, antibody-dependent cellular killing and stimulation of apoptosis [25]. Rituximab can suppress all stages of B lymphocyte differentiation except stem cells and long-lived plasma cells. The majority of pharmacokinetic and pharmacodynamic studies have been performed in patients with B-cell lymphoma. In 9 patients given 375 mg/m2 rituximab as an intravenous infusion in four doses, mean serum half-life was 59.8 hours after the first infusion and 174 hours after the fourth infusion [26]. However, no pharmacokinetic or pharmacodynamics data on rituximab are available in subjects with end-stage liver disease. Therefore, we do not know how much, how often or when rituximab should be administered for DSZ in ABOi LT. Most programs have adopted the original dosage used in malignant lymphoma: 375 mg/body surface area (BSA). Administration was started 1 to 3 weeks prior to transplantation, and the frequency of dosing was titrated from multiple to single dosing after there was found to be significantly higher rates of infectious complications and profound long-standing leukopenia with multiple dosing. Several clinical studies of the effectiveness of lower dosage have been performed in ABOi KT [27]. One Korean center has reported success with a lower rituximab dose in ABOi KT [28].
Egawa et al. [29] have carried out a very important study of the impact of rituximab DSZ on ABOi LDLT. By December 2011, the Japanese registry contained clinical and laboratory data on 663 patients who had undergone ABOi LDLT in 37 institutions. Of these patients, 381 were adults. The incidence of AMR decreased from 23.5% to 6.2% after the use of rituximab. According to that study, the timing, number of doses and total dose did not affect patient survival or the incidence of AMR. Moreover additional modification of the DSZ regimen to suppress B-cell activity or inflammatory responses failed to have a positive impact; multiple doses of rituximab and additions to the DSZ regimen such as SPL or LGIT increased the incidence of fungal and CMV infection, and the usual rituximab dose of 375 mg/BSA had an adverse impact on the incidence of infection in comparison with a smaller dose. There is a need to develop a consensus on rituximab dosage, frequency and timing in ABOi LT based on large cohort studies.
The survival outcomes of ABOi LT largely depend on the pre-transplant patients' condition and age. A Japanese multicenter retrospective study reported that pre-transplant intensive care unit stay and high MELD score were the most significant risk factors for patient survival [10]. The most common cause of death was not AMR but infectious complications, which were attributed to potent immunosuppression with high-dose steroid, calcineurin inhibitor or a combination of cell-depleting agents, etc. Therefore, prudent patient selection as well as prevention of AMR are crucial factors for successful outcomes in ABOi LT.
Age is also a powerful indicator of survival outcomes [30]. The immune system is usually immature in children, especially in infants. In the latter, the titer of IA is very low or zero, and B-cell responses to antigenic stimuli are very weak. Therefore, the incidence of AMR in infants was very low even before the era of rituximab. Survival after ABOi LDLT was comparable with after ABOc, without any special measures to prevent AMR. However clinical outcomes deteriorate with increasing patient age, generally starting at age 8.
Pretransplant and post-transplant peak IA titers may be significant risk factors for AMR. In that connection, recipient blood type O has been considered a risk factor for AMR because IA titers are intrinsically higher in type O than in other blood types [10]. However, a high pre-transplant IA titer can be lowered by repetitive TPE and high dose intravenous immunoglobulin, and the post-transplant rebound rise of IA can be efficiently suppressed by rituximab prophylaxis and post-transplant TPE. Thus, the impact of IA titer upon AMR has been diminished, and no relationship with patient survival has been found in recent studies, nor is the recipient's blood type or mismatch pattern related to AMR or patient survival.
The epoch-making event in ABOi LT was the introduction of rituximab for DSZ, so that the history of ABOi LT can be divided into before, and after, rituximab. A Japanese nationwide survey revealed that 3-year survival increased from 30% to 80% after the introduction of rituximab [31] and a recent Japanese multicenter study revealed that the only relevant risk factor for AMR was a DSZ protocol without rituximab [30]. In the most recent large single center cohort including 142 adult patients undergoing ABOi ALDLT, Song et al. [32] obtained 3-year graft and patient survival rates of 90.9% and 96.3%, respectively. In the comparative analysis of survival outcome from the single center experience including 235 ABOi and 1,301 ABOc LDLD recipients from November 2008 to December 2013, the results were comparable. The 1, 3, and 5-year graft survival rate of ABOi group was 93.3, 89.2, and 89.2%, respectively and the 1, 3, and 5-year patient survival rate for ABOcwas 96.5, 92.3, and 92.3%, respectively. There was no significant difference in the graft and patient survival rate between the two groups (Fig. 3). These survival outcomes represented a significant impro-vement on previous studies. In this study, all the patients received pretransplant rituximab prophylaxis, the mean MELD score was 12.7 and no patients with pre-transplant ICU stay were included. From those findings, we can conclude that patient selection and effective DSZ by rituximab are the most important factors contri-buting to successful outcomes in ABOi LT.
Despite the recent remarkable improvement in survival outcomes in ABOi LT, there still exist serious concerns about intractable biliary complications. In general, graft failure in connection with ABO incompatibility can take two clinical forms: (1) 'hepatic necrosis' occurring within one or two weeks and leading to massive graft necrosis within a month, (2) DIHBS occurring several months after LT, with development of diffuse and multiple strictures of the intrahepatic bile duct. Since the introduction of rituximab, however, the fulminant type of hepatic necrosis occurring soon after ABOi ALDLT has nearly disappeared and DIHBS has become the main risk factor for graft failure. The prognosis of DIHBS is very poor because refractory cholangitis leads to sepsis and graft failure, although mortality is not 100% as in fulminant hepatic necrosis. DIHBS is an unpleasant and dangerous complication because it cannot usually be resolved by conventional biliary interventions. The only proven treatment is re-transplantation. Furthermore, the occurrence of DIHBS significantly affects patient's quality of life because of repeated cholangitis, biliary intervention and re-admissions. According to several studies, high pre-LT and post-LT levels of ADA are closely related to the occurrence of AMR [10,14,30,31,33] In the study by Song et al., DIHBS occurred exclusively in patients (12 patients; 8.5%) receiving ABOi ALDLT. Three deaths and 4 cases of re-transplantation were related to DIHBS, and graft and patient survival rates were significantly reduced in the ABOi ALDLT recipients with DIHBS. However, these workers failed to identify any significant risk factors for DIHBS. Thus, the next step in further improving survival outcomes in ABOi LT is to elucidate the risk factors for DIHBS and identify preventive or therapeutic measures.
In Korea, the first ABOi LT was perfomed in Februrary 1996. The patient was a 6-year-old girl, who is still alive. The application ABOi LT in the adult population of Korea was delayed until 2007 because of concern about the poor recipient survival outcomes in the early Japanese experience. Unfortunately, the initial experience in Korea was also not promising [34]. However, the modifications of the DSZ protocol and careful patient selection since 2008 have yielded encouraging results [32,35,36]. By 2013, the number of ABOi ALDLT programs in Korea had increased to 11 (Fig. 4A). A total of 399 ABOi ALDLT were performed in Korea between 2007 and 2013, accounting for 7.5% of all ALDLT; this percentage had increased to 18% by last year (Fig. 4B), which demonstrates that ABOi donors have had a real impact on the donor pool. In the Korean experienceall the patients received rituximab prophylaxis. The 3-year graft and patient survival rates were 86.5 and 87.6%, respectively, over a mean of 20.8 months of follow-up. In the full data set of 307 cases in 8 Korean centers, 26 (8.5%) of the patients experienced AMR. Of these, only 2 suffered hepatic necrosis due to full-blown AMR. Detailed clinical information on the 26 patients with AMR is given in Table 1 and the outcomes are depicted in Fig. 5. Graft and patient survival rates were greatly affected by concomitant AMR (Fig. 6). In a univariate analysis of the Korean experience, antecedent acute cellular rejection, high initial IA titer (≥512) and high post-transplant peak IA (≥512) were significant risk factors. However, we failed to identify any meaningful risk factors in multivariate analysis.
The use of ABOi living liver donors is a very effective and safe method for expanding the donor pool in LDLT. In the Korean experience, ABOi living liver donors have had a great impact on the liver donor pool, and the outcomes of ABOi ALDLT are now encouraging. Therefore, transplant surgeon should consider the applicability of ABOi LDLT in the case of no available ABOc living donor, although there has not been concrete guideline for the recommendation. However, there still remains concern about DIHBS, an attenuated form of AMR. Ultimately, we need to identify definite risk factor for DIHBS and preventive measures against it by further studies focusing on B and T-lymphocyte activities and adaptation mechanism in ABOi LT.
Figures and Tables
 | Fig. 1Anuual number of liver transplants in Korea (A) and cumulative number on the waiting list for deceased donor liver transplantation together with the annual number of deceased donor liver transplants (B). LDLT, living donor liver transplants; KONOS, Korean Network for Organ Sharing. |
 | Fig. 2Pre-transplant desensitization and post-transplant immunosuppression protocols for ABO-incompatible liver transplantation. LDLT, living donor liver transplants. |
 | Fig. 3Comparison of graft (A) and patient (B) survival in 235 ABO-incompatible and 1,301 ABO-compatible adult living donor liver transplant recipients. |
 | Fig. 4Number of ABO-incompatible adult living donor liver transplantation programs (A) and annual number of ABO-incompatible adult living donor liver transplants (B) in Korea. |
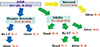 | Fig. 5The fates of the 26 patients experiencing antibody-mediated rejection among 307 cases of ABO-incompatible adult living donor liver transplants in 8 Korean centers. AMR, antibody-mediated rejection; DIHBS, diffuse intrahepatic biliary stricture. |
 | Fig. 6Comparison of graft (A) and patient (B) survival rates in ABO-incompatible adult living donor liver transplant recipient with and without diffuse intrahepatic biliary stricture. |
References
1. Kuramitsu K, Fukumoto T, Iwasaki T, Tominaga M, Matsumoto I, Ajiki T, et al. Long-term complications after liver transplantation. Transplant Proc. 2014; 46:797–803.

2. Gordon RD, Iwatsuki S, Esquivel CO, Tzakis A, Todo S, Starzl TE. Liver transplantation across ABO blood groups. Surgery. 1986; 100:342–348.
3. Gugenheim J, Samuel D, Reynes M, Bismuth H. Liver transplantation across ABO blood group barriers. Lancet. 1990; 336:519–523.

4. Demetris AJ, Jaffe R, Tzakis A, Ramsey G, Todo S, Belle S, et al. Antibody-mediated rejection of human orthotopic liver allografts. A study of liver transplantation across ABO blood group barriers. Am J Pathol. 1988; 132:489–502.
5. Tanaka A, Tanaka K, Kitai T, Yanabu N, Tokuka A, Sato B, et al. Living related liver transplantation across ABO blood groups. Transplantation. 1994; 58:548–553.

6. Tanabe M, Shimazu M, Wakabayashi G, Hoshino K, Kawachi S, Kadomura T, et al. Intraportal infusion therapy as a novel approach to adult ABO-incompatible liver transplantation. Transplantation. 2002; 73:1959–1961.

7. Nakamura Y, Matsuno N, Iwamoto H, Yokoyama T, Kuzuoka K, Kihara Y, et al. Successful case of adult ABO-incompatible liver transplantation: beneficial effects of intrahepatic artery infusion therapy: a case report. Transplant Proc. 2004; 36:2269–2273.

8. Usuda M, Fujimori K, Koyamada N, Fukumori T, Sekiguchi S, Kawagishi N, et al. Successful use of anti-CD20 monoclonal antibody (rituximab) for ABO-incompatible living-related liver transplantation. Transplantation. 2005; 79:12–16.

9. Egawa H, Ohdan H, Haga H, Tsuruyama T, Oike F, Uemoto S, et al. Current status of liver transplantation across ABO blood-type barrier. J Hepatobiliary Pancreat Surg. 2008; 15:131–138.

10. Egawa H, Teramukai S, Haga H, Tanabe M, Fukushima M, Shimazu M. Present status of ABO-incompatible living donor liver transplantation in Japan. Hepatology. 2008; 47:143–152.

11. Tanabe M, Kawachi S, Obara H, Shinoda M, Hibi T, Kitagawa Y, et al. Current progress in ABO-incompatible liver transplantation. Eur J Clin Invest. 2010; 40:943–949.

12. Raut V, Uemoto S. Management of ABO-incompatible living-donor liver transplantation: past and present trends. Surg Today. 2011; 41:317–322.

13. Haga H, Egawa H, Shirase T, Miyagawa A, Sakurai T, Minamiguchi S, et al. Periportal edema and necrosis as diagnostic histological features of early humoral rejection in ABO-incompatible liver transplantation. Liver Transpl. 2004; 10:16–27.

14. Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Williams WW, Tolkoff-Rubin N, et al. Complement activation in acute humoral renal allograft rejection: diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol. 1999; 10:2208–2214.
15. Behr TM, Feucht HE, Richter K, Reiter C, Spes CH, Pongratz D, et al. Detection of humoral rejection in human cardiac allografts by assessing the capillary deposition of complement fragment C4d in endomyocardial biopsies. J Heart Lung Transplant. 1999; 18:904–912.

16. Magro CM, Pope Harman A, Klinger D, Orosz C, Adams P, Waldman J, et al. Use of C4d as a diagnostic adjunct in lung allograft biopsies. Am J Transplant. 2003; 3:1143–1154.

17. Haga H, Egawa H, Fujimoto Y, Ueda M, Miyagawa-Hayashino A, Sakurai T, et al. Acute humoral rejection and C4d immunostaining in ABO blood type-incompatible liver transplantation. Liver Transpl. 2006; 12:457–464.

18. Kozaki K, Egawa H, Ueda M, Oike F, Yoshizawa A, Fukatsu A, et al. The role of apheresis therapy for ABO incompatible living donor liver transplantation: the Kyoto University experience. Ther Apher Dial. 2006; 10:441–448.

19. Thalgahagoda S, Webb NJ, Roberts D, Birch A, Milford DV, Tavakoli A, et al. Successful ABO incompatible renal transplantation following rituximab and DFPP after failed immunoadsorption. Pediatr Transplant. 2014; 18:E74–E76.

20. Eskandary F, Wahrmann M, Biesenbach P, Sandurkov C, Konig F, Schwaiger E, et al. ABO antibody and complement depletion by immunoadsorption combined with membrane filtration--a randomized, controlled, cross-over trial. Nephrol Dial Transplant. 2014; 29:706–714.

21. de Weerd AE, van Agteren M, Leebeek FW, Ijzermans JN, Weimar W, Betjes MG. ABO-incompatible kidney transplant recipients have a higher bleeding risk after antigen-specific immunoadsorption. Transpl Int. Forthcoming 2014.

22. Ikegami T, Taketomi A, Soejima Y, Yoshizumi T, Uchiyama H, Harada N, et al. Rituximab, IVIG, and plasma exchange without graft local infusion treatment: a new protocol in ABO incompatible living donor liver transplantation. Transplantation. 2009; 88:303–307.

23. Song GW, Lee SG, Hwang S, Ahn CS, Kim KH, Moon DB, et al. Section 15. A desensitizing protocol without local graft infusion therapy and splenectomy is a safe and effective method in ABO-incompatible adult LDLT. Transplantation. 2014; 97:Suppl 8. S59–S66.

24. Raut V, Mori A, Kaido T, Ogura Y, Taku I, Nagai K, et al. Splenectomy does not offer immunological benefits in ABO-incompatible liver transplantation with a preoperative rituximab. Transplantation. 2012; 93:99–105.

25. Pescovitz MD. Rituximab, an anti-cd20 monoclonal antibody: history and mechanism of action. Am J Transplant. 2006; 6:859–866.

26. Ramos EJ, Pollinger HS, Stegall MD, Gloor JM, Dogan A, Grande JP. The effect of desensitization protocols on human splenic B-cell populations in vivo. Am J Transplant. 2007; 7:402–407.

27. Toki D, Ishida H, Horita S, Setoguchi K, Yamaguchi Y, Tanabe K. Impact of low-dose rituximab on splenic B cells in ABO-incompatible renal transplant recipients. Transpl Int. 2009; 22:447–454.

28. Chung BH, Hong YA, Sun IO, Piao SG, Kim JI, Moon IS, et al. Determination of rituximab dose according to immunologic risk in ABO-incompatible kidney transplantation. Ren Fail. 2012; 34:974–979.

29. Egawa H, Teramukai S, Haga H, Tanabe M, Mori A, Ikegami T, et al. Impact of rituximab desensitization on blood-type-incompatible adult living donor liver transplantation: a Japanese multicenter study. Am J Transplant. 2014; 14:102–114.

30. Egawa H, Oike F, Buhler L, Shapiro AM, Minamiguchi S, Haga H, et al. Impact of recipient age on outcome of ABO-incompatible living-donor liver transplantation. Transplantation. 2004; 77:403–411.

31. Kawagishi N, Satomi S. ABO-incompatible living donor liver transplantation: new insights into clinical relevance. Transplantation. 2008; 85:1523–1525.

32. Song GW, Lee SG, Hwang S, Kim KH, Ahn CS, Moon DB, et al. Biliary stricture is the only concern in ABO-incompatible adult living donor liver transplantation in the rituximab era. J Hepatol. 2014; 61:575–582.

33. Uchiyama H, Mano Y, Taketomi A, Soejima Y, Yoshizumi T, Ikegami T, et al. Kinetics of anti-blood type isoagglutinin titers and B lymphocytes in ABO-incompatible living donor liver transplantation with rituximab and plasma exchange. Transplantation. 2011; 92:1134–1139.

34. Kim BW, Park YK, Kim YB, Wang HJ, Kim MW. Effects and problems of adult ABO-incompatible living donor liver transplantation using protocol of plasma exchange, intra-arterial infusion therapy, and anti-CD20 monoclonal antibody without splenectomy: case reports of initial experiences and results in Korea. Transplant Proc. 2008; 40:3772–3777.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download