Abstract
Heart transplantation is the last treatment option in refractory end stage heart failure, which can prolong survival. The number of heart transplantations has increased and the survival rate has improved during the last few decades which was contributed by advanced understanding of immunologic mechanism of rejection, pharmaceutical development and clinical management of donors and recipients. However, only a fraction of patients can be offered to transplantation due to shortage of donor heart and many patients suffer high mortality while waiting. Meanwhile, technical advancement of mechanical assist device in recent years enabled long term implantable left ventricular assist devices (LVAD) to bridge the patients with high mortality in the waiting list to transplantation and to assist as a long term destination therapy for patients who are not eligible for transplantation. Development of solid phase assay increased the sensitivity and the specificity of detection of anti-human leukocyte antigen (HLA) antibodies in the recipient. It enabled identifying unacceptable HLA antigens, acquire calculated Panel Reactive Antibodies and perform virtual cross match that can enhance the efficacy of donor allocation system to decrease the waiting time, obviate prospective cross match to decrease ischemic time and to assess the risk of rejection in presensitized patients. Antibody mediated rejection is a challenging entity in diagnosis and management. However, standardized classification of histology and immunology of endomyocardial biopsies was made recently and immunotherapy is moving toward targeted therapies directed at antibody production and function. This review focuses on those major changes in the heart transplantation field in the last decade.
The prevalence of acute and chronic heart failure is increasing despite improvement in medical, interventional and surgical treatment modalities. Since the first human heart transplantation was performed in 1967, the number of heart transplantations has increased remarkably worldwide, which was contributed by advancement in understanding immunology and rejection, pharmaceutical development and clinical management of the donors and recipients. Donor shortage is the major factor limiting the transplantation rate nowadays. Heart transplantation was the only option for refractory end stage heart failure to allow durable survival, until recently. However, technical advancement of left ventricular assist device (LVAD) made its application feasible in daily practice and improved survival comparable to transplantation and emerged as another valuable option for long term cardiac assist in transplant ineligible patients.
Transplantation rate has been increased since 1980s until its peak in 1990s, when it became more stable in recent days. More than 4,000 heart transplantations were done in 2011 in over 200 centers around the world as reported by ISHLT (The International Society for Heart and Lung Transplantation) (Fig. 1A). Regarding 103, 299 cases of pediatric and adult heart transplants which were undergone between 1982 and 2011 in the world, 1-year survival rate was 81%, 5-year survival rate 69%, median survival rate 11 years for all the patients and 13 years for those surviving after the first year of transplantation according to ISHLT report (Fig. 2A)[1]. It is clear that heart transplantation is now a valuable treatment option for refractory end stage heart failure.
Survival rate improved continuously over the last 3 decades which was mostly contributed by reduction of mortality in the first-transplant year. Risk factors for 1-year survival are underlying causes for heart failure, donor and recipient age, recipient Body Mass Index (BMI), donor and recipient BMI ratio, allosensitization status of recipient, clinical status of recipient prior to transplantation, such as hospitalization and inotropic support, ventilator support, mechanical circulatory support (MCS), co-morbidities of recipient such as infection, diabetes mellitus (DM), renal dysfunction, liver dysfunction, high pulmonary vascular resistance, and malignancy. History of previous open heart surgery of recipient, ischemic time and volume of transplant center are the significant procedure related variables that affect early outcome. Risk factors for five year and long term survival are similar but additional factors such as number of rejection, non-use of calcineurin inhibitors (CNI), cardiac allograft vasculopathy (CAV), retransplantation, become significant [1].
Regarding underlying etiology of heart failure, dilated cardiomyopathy and ischemic cardiomyopathy are the two most common causes of transplantation which showed favorable prognosis compared to congenital heart disease and retransplantation in early survival. However, congenital heart disease is not a risk factor any more for long term survival.
Heart transplantation can be considered in the advanced heart failure patients who are in critical condition with no other long term option or in chronically refractory to conventional treatment and inotrope dependent patients or in ambulatory but frequently hospitalized patients due to decompensated heart failure. To better evaluate the ambulatory heart failure patients, VO2max is an extremely valuable parameter which can assess cardiac output indirectly and noninvasively in response to exercise. Moreover, other risk factors should be considered or avoided to achieve the best result possible which are comorbidities such as advanced DM, irreversible renal, hepatic and pulmonary dysfunction (kidney, liver, lung co-transplantation can be considered), fixed pulmonary vascular resistance >6 Wood unit, acute infection (beside infection associated with VAD which can only be treated by device removal), past history of malignancy in addition to age, BMI, socioeconomic and psychological aspects. However, no single parameter can determine the selection of the transplantation candidates, instead all of the factors should be considered. Several risk assessment models such as HFSS (Heart Failure Survival Score), SHFM (Seattle Heart Failure Model), ADHERE study, and OPTIME-CHF study are available for stratification of the risk and prognosis, and to assist in the selection of appropriate candidates for heart transplantation [2]. Above all, clinical discretion based on careful assessment of risk factors is the mandatory initial step for successful heart transplantation.
When a donor heart becomes available, a heart transplantation allocation system such as the OPTN/UNOS (Organ Procurement and Transplantation Network/United Network of Organ Sharing) in US, the Eurotransplant in EU and the KONOS (Korean Network for Organ Sharing in Korea would match the candidate by the rank prioritized by medical urgency, accumulated waiting time, blood group compatibility, distance or zone from donor hospital (Table 1) [3,4,5].
In Korea, a total of 903 heart transplantations were done by December 2013 since its first performance in 1992. It increased steadily from less than 30 cases a year by the mid 2000s to more than 100 cases annually since 2011 (Fig. 1B). However, the waiting list for transplantation increased sharply compared to the transplantation rate in the same period. Dilated cardiomyopathy is the most common cause of transplantation which comprises 56% of all transplantations, which is followed by ischemic cardiomyopathy in 16%. KONOS status 0 and 1 which corresponds to UNOS 1A comprised about 50% of transplantations and about 11% was bridged by mechanical circulatory support during 2006 to 2012 mostly with IABP (Intra-Aortic Balloon Pump) and ECMO (Extra-Corporeal Membrane Oxygenation) (Table 1) [4,5]. For 658 adult heart transplant recipients who were followed up for a median of 47 months (range 21.1-93.7 months), median survival rate was 18.5 years, 1 year survival rate of 87%, 5 year survival rate of 76%, 10 year survival rate of 64% which was comparable to or surpassing ISHLT data (Fig. 2B). Cause of death was similar to ISHLT data, of which the most common cause was graft failure in the first month, infection was the most common cause after the first month during the first year, rejection peaked in the first to third post transplantion year and malignancy and CAV became the major cause of death thereafter [6,7,8].
REMATCH trial in 2001 was the first study that showed long term survival benefit of the first generation pulsatile pump LVAD (Heart Mate XVE®, Thoratec Co, USA) over medical treatment in refractory heart failure who were ineligible for transplantation) [9]. In 2008 and 2010, second generation continuous flow axial pump LVAD (Heartmate II®, Thoratec Co, USA) was approved by the US FDA as a long term implantable device for bridge to transplant (BTT) and destination therapy (DT) by improved durability and significantly less side effects compared to the pulsatile pumps [10,11]. Third generation continuous flow centrifugal pump LVAD (HVAD®, HeartwareInc, USA) has the advantage of pocketless, bearingless smaller size pump which can avoid pocket infection and is suitable for patients of small body habitus. It was approved for BTT in 2012 [12] and clinical trials for DT is ongoing [13]. Since 2010 when HM II was approved for DT, more than 95% of DT patients have been implant ed with continuous flow LVAD.
Compared to stable transplantation rate, implantation of LVAD increased sharply in recent years. More than one third of transplantations are now bridged by LVAD and more than 40% of all the LVAD implantation in US are performed for DT according to INTERMACS (The Interagency Registry for Mechanically Assisted Circulatory Support, USA) (Fig. 1C) [14]. The number of BTT also increased and improved the survival rate of transplant candidates in waiting list [15]. On the other hand, there is concern that increase of VAD complications which accompany increase in BTT such as systemic or local infection, bleeding from coagulopathy, throm bembolic events, pump failure and presensitization of candidates, would render the candidates to more prolonged waiting time and complicated transplantation. However, after the debate over the post- transplantation survival with BTT [16], the short and long term survival rate in durable continuous flow LVAD era seem to be comparable to that of heart transplantation without LVAD except for the transplantations with few complications such as device infection [17,18].
Nowadays LVAD is indicated in patients who are chronically or acutely decompensated and dependent on high dose of inotropes or intolerable to inotropes or who are frequently hospitalized (INTERMACS 2-4) for BTT or DT. They should fulfill the inclusion criteria of transplantation but several risk factors which may have precluded them from listing such as age, obesity, pulmonary hypertension, recent malignancy, HIV infection and moderate renal or hepatic dysfunction are permitted in LVAD [19].
In the current era of continuous flow LVAD, 1 year survival rate is 80%, 2 years survival rate is 70%. LVAD for DT has slightly higher risk than BTT (Fig. 2C) [20,22]. Freedom from adverse events is about 30% at 1 year and quality of life measures are generally positive for at least the first year after implantation. Old age (especially over 70), INTERMACS level (1 or 2 vs others), BTT vs DT, renal dysfunction (severe dysfunction requiring dialysis), right ventricular dysfunction (especially requiring Biventricular Assist Device [BiVAD]), surgical complexity (prior cardiac surgery and concomitant cardiac procedures) are the risk factors for early mortality [14]. With durable continuous flow LVAD, early postoperative mortality is not related with device malfunction, instead it was related to patient factors which emphasizes the importance of selection of appropriate patients and timing of implantation.
Most of the implantations were done in INTERMACS level 1-2 in the early LVAD era which was too critical to expect the best benefit of LVAD, so the trend nowadays is to implant in an earlier stage before development of irreversible end organ dysfunction, right ventricular failure, or cardiogenic shock. However, too early implantation may not justify the possible adverse effects of LVAD. There are several risk scoring systems to predict postoperative or long term survival after VAD implantation, however, art of clinical judgment along with comprehensive analysis with scoring system is critical. In the setting of INTERMACS stage 1-2, temporary mechanical circulatory support (MCS) such as VA ECMO, IABP, short-term VAD such as CentriMag® VAD (Thoratec Co, USA), percutaneous LVAD such as Impella® (AbioMedInc, USA), Tandem Heart® (CardiacAssistInc, USA) are indicated immediately for bridge to decision (BTD) and are evaluated for candidacy for long term strategy as soon as MCS is placed (Table 2) [21,22,23].
Depending on the change of patient's clinical status after LVAD implantation such as resolved pulmonary hypertension, improved renal and hepatic function, decreased overweight, etc., patient's management strategy can change. In contrary, adverse events after VAD may delist the patient from transplantation candidacy. So designation of BTT, DT, BTD are interchangeable rather than permanent.
Common complications of VAD, which are bleeding, arrhythmia, infection, thromboembolic event, pump failure, and aortic insufficiency decreased meaningfully when compared to pulsatile LVAD. However, there are still limitations in long term survival and quality of life which results in frequent rehospitalization and economic burden. About 18-29% of cost was added to the initial budget by readmission due to adverse events [24]. Cost effectiveness is not yet high with LVAD in this era and it is one of the important reasons to select the most appropriate patient in the appropriate time.
Presensitization may result from previous transfusion, pregnancy, prior transplantation, prior cardiac surgery with homograft, infection and VAD and it is the major cause of hyperacute rejection, acute rejection, antibody-mediated rejection (AMR), long term complication of CAV, decreased survival and prolonged waiting time to transplantation [25,26].
Donor specific antibodies (DSA) can be avoided by crossmatching recipient's serum with potential donor cells prior to transplantation. However, routine prospective crossmatching is not always possible in heart transplantation because of limited ischemic time. So the need for prospective crossmatching is usually decided by the presensitization status of recipient which is measured by Panel Reactive Antibody (PRA). PRA is based on traditional complement-dependent cytotoxicity method which is the percentage of positive crossmatch when the recipient serum is reacted with various anti-HLA antigens from various donors of the region. It is the measure of allosensitization of the transplant candidate, which may reflect how difficult the patient can find a donor heart and how much risk of rejection the recipient may have after transplantation [27]. However, the drawback is that the panel of antigens being tested is not a perfect representation of the donor pool, the cutoff where sensitization is defined and the way of reporting PRA (peak PRA vs PRA prior to transplantation) is not standardized and the crossmatch can detect both anti-HLA Ab and non-HLA Ab of which clinical relevance is not defined yet.
Meanwhile, solid phase assay (SPA) using single HLA antigen (Ag) attached to bead (Luminex®, OnelambdaInc, USA) has been developed in the last decade which enabled more sensitive, more specific identification and quantification of affinity of anti-HLA Abs. This technology enabled identification of high titer anti-HLA Abs in recipient against potential donor HLA Ag which is called "the unacceptable Ag" and based on this technology, calculated PRA (cPRA) and virtual crossmatch are developed to find a proper donor in a shorter waiting time and has enabled the avoidance of a prospective crossmatch. cPRA is originally derived from kidney donors in the US between 2003 and 2005 and it represents the percentage of organ donors that express one or more unacceptable anti-HLA Ags in the donor pool, thus allowing the prediction of the probability of finding a donor heart without unacceptable Ag [28]. Virtual crossmatch compares the HLA Ag of donor and HLA Ab of recipient virtually before transplantation, which can avoid prospective crossmatching and save time to allow an acceptable ischemic time until the donor heart arrives; thus enabling an increase in the available donor pool [29]. Defining the cutoff of unacceptable Ag is upto each center, which is generally more than 5,000-10,000 MFI (Mean Florescence Intensity); but it also depends on the ability and willingness of the center to deal with high risk transplantation (Fig. 3).
Surprisingly, there are few observational studies, but no randomized controlled trials that studied the benefit of desensitization in presensitized patients. Many centers practice desensitization therapies with various combinations of intravenous immunoglobulin (IVIg), plasmapheresis (PP), cyclophosphamide, rituximab to decrease burden of DSA in presensitized candidates. However, there are no consensus on protocol, clinical goal and benefit of desensitization, yet.
Schaffer et al. retrospectively analyzed a large number of transplant recipients before 2004 comparing the group of presensitized patients,whose peak PRA higher than 20%, significantly reduced after densensitization treatment with the group of presensitized patients with trivial reduction of PRA. They reported the peak PRA and percent reduction of PRA before transplantation are the most significant prognostic factors that made a significant graft survival difference in the first 30 days after transplantation, although 10 year long term survival was worse than nonsensitized patients [30].
Kobashigawa et al. showed comparable 5 years survival rate and CAV rate among group of patients with highest pretransplant PRA with desensitization (average PRA 70.5±28.9%) and patients with moderately high PRA without desensitization (average PRA 18.8±8.5%) and group of non-sensitized patients (average PRA 0.9±2.2%) [31], which showed benefit of desensitization therapy.
PP aims to remove preformed antibodies, but it is usually combined with IVIg or other B cell suppressive drugs to block antibody rebound. There are small observational studies with various combination of drugs that showed significant decrease of the risk of rejection and survival rate compared to non-sensitized patients. But, it is an invasive procedure and carries risk of infection.
IVIg is a pool of IgG from random donors and it has immunomodulatory effect in high dose (1-2 g/kg) by various mechanisms such as direct cytokine inhibition, Fc receptor binding, C3 blockade, and modulating anti T cell, B cell, and APC activity. There are various timing and dosing schedules for PP and IVIg among transplant centers.
Rituximab is a chimeric monoclonal Ab against B cell receptor CD20, which is primarily used in B cell neoplasm. It has limited experience in heart transplantation but a small series coming from renal transplantation was shown effectiveness for desensitization and treatment of AMR along with IVIg [32,33].
Bortezomib is a 26S proteasome inhibitor approved in multiple myeloma and lymphoma which decreases production of alloantibodies by plasma cells and induces cell apoptosis. It has been reported to be effective in a few cases of desensitization and treatment of AMR in heart and kidney co-transplantation [34,35].
Although there are still controversies over indication, clinical effect, timing or dosing strategy of induction immunotherapy, it is widely practiced especially in subpopulations such as primary graft failure, presensitized patients and poor renal function who can delay starting of CNI by induction therapy. Due to the risk of hypersensitivity and infection, OKT3 (murine anti-CD3 monoclonal Ab) is withdrawn from the clinical arena and replaced by polyclonal agents such as thymoglobulin (rabbit anti-thymocyte globulin (ATG), Genzyme Co, USA) and ATGAM (equine ATG, Pfizer, USA).
Basiliximab (Simulect, NorvartisPharma AG, Swiss) and daclizumab (Zenapax, Roche, Swiss) are anti CD25 monoclonal Abs which decrease the risk of acute rejection when added to standard triple regimen in heart transplant recipients compared to patients without induction therapy in a randomized clinical trial [36,37]. In contrast to ATG with the risk of infection, anti CD25 Abs have a good safety profile. However, daclizumabhas been withdrawn from the market for commercial reasons.
Alemtuzumab (Campath-1H, Genzyme Co, USA) is humanized monoclonal anti CD52 Ab which showed profound and long lasting lymphocyte suppression when used as induction therapy and steroid resistant rejection in mainly renal transplantation, but it has very limited study in heart transplantation and has been with drawn from the market.
Eculizumab (Soliris, Alexion, USA) is humanized monoclonal Ab against complement 5 which inhibits cleavage of C5 into C5a and C5b which is a component of the membrane attack complex (MAC) of the classical pathway. It has been shown to reduce AMR in renal transplantation but no evidence has been seen in heart transplantation yet.
Endomyocardial biopsy (EMB), therapeutic drug monitoring (TDM), serology for donor specific Abs (DSA), and echocardiography are all done periodically for surveillance of graft function and early sign of rejection, especially in the first year of transplantation; however, no one method can replace all the others.
EMB is the sine qua non essential tool for the diagnosis of acute and chronic rejection at present since the diagnostic criteria is based on pathologic findings. However, it is an invasive procedure which has low but definite side effects and has the probability of missing the patch distribution of rejection. The pathologic findings appear in the late stage, so there is much effort made to replace or reduce EMB with noninvasive sensitive and specific tests. In the CARGO study, genetic markers of moderate/severe rejection were derived and validated with microarray technology. It showed good agreement with ISHLT≥3A cellular rejection and was able to avoid EMB in the low rejection risk population after 6 months to 1 year of transplantation [38,39].
Graft systolic function should be monitored through regular echocardiography along with EMB and serum DSA, but as systolic dysfunction is a late manifestation of rejection, clinical suspicion and immunopathologic findings are important for early diagnosis of rejection [40].
The cornerstone of longterm success of transplantation is the management of immunosuppressive drugs which are to prevent or to treat rejection and minimize complication.
Introduction of cyclosporine in the early 1980s was a milestone in transplantation history which decreased rejection related mortality remarkably compared to the time prior to cyclosporine. Introduction of tacrolimus (FK 506) in the 1990s seems to have superior prevention in acute rejection with comparable risk of renal dysfunction and infection with cyclosporine, although there was no difference in 1 year survival rate [41,42].
Mycophenolate Mofetil (MMF) showed superior prevention of acute rejection and better long term survival by reducing CAV compared to azathioprine when added to cyclosporine or tacrolimus [43,44]. MMF seems to ameliorate the side effects of CNI of hypertension, hyperglycemia, hyperlipidemia and renal dysfunction by reducing CNI doses without compromising immunosuppressive effect which makes it a useful combination with CNI. In terms of malignancy, azathioprine seems to have higher risk of developing malignancy than MMF when combined with cyclosporine. Tacrolimus has less risk of malignancy than cyclosporine when combined with MMF. Since the 1990s long term survival increased with decrease in acute and chronic rejection as tacrolimus and MMF replaced cyclosporine and azathioprine.
PSI/mTOR inhibitor (Proliferation signal inhibitor/mammalian target of rapamycin inhibitor) sirolimus and its derivative everolimus have been more recently introduced and they are more effective in reducing acute rejection than azathioprine in combination with cyclosporine. Everolimus showed similar risk of acute rejection compared with MMF in combination with cyclosporine [43,45,46]. Sirolimus had similar risk of acute rejection compared with MMF but had more side effects when combined with tacrolimus [47]. PSI/mTOR inhibitors decrease the incidence of CAV and maximal intimal thickness in IVUS (Intravascular Ultrasound) by inhibiting smooth muscle proliferation and decreasing CMV infection compared to azathioprine or MMF, which is independent of CMV prophylaxis and donor/recipient CMV serostatus [46]. Converting maintenance regimen from standard CNI to everolimus with reduced CNI offers significant improvement in renal function, although switch back to standard CNI may be occasionally needed because of side effects of PSI/mTOR inhibitors such as delayed wound healing, severe proteinuria, severe hyperlipidemia and potential infection. However, renal benefit was not proven in de novo PSI/mTOR inhibitor with CNI regimen in comparison to MMF with CNI yet [48]. PSI/mTOR inhibitor has antineoplastic effect by PI3K pathway and p53 pathway to sensitize the tumor cell to apoptosis and everolimus is a certified agent for advanced renal cell carcinoma and breast cancer. There are case reports on regression of Kaposi sarcoma, lymphoma, non-melanoma skin cancer and renal cell cancer with everloimus in renal and heart transplantation. Tacrolimus in combination with everolimus seem to be as effective in immunosuppression as cyclosporine, but evidence is limited.
Considering their high efficiency in preventing acute rejection, CNI based regimens are still the cornerstone of maintenance therapy. However, due to notorious nephrotoxicity, CNI minimization or CNI free maintenance protocol have been studied to preserve renal function and the immunological safety at the same time. There are ample studies comparing MMF or PSI/mTOR inhibitor with decreased CNI dose switched from azathioprine with standard CNI dose, and non-switched patients, reporting improved renal function and immunologically safe outcome. But there are no data on cyclosporine target level where renal and immunological outcomes are both met.
There are few studies on CNI free maintenance regimen, which further pursue renal preservation but there are still controversial on the efficacy, safety and the time when CNI should be withdrawn. CNI free regimen during the first post transplantion year is not unanimously recommended and there are few pilot studies on CNI free regimen from the onset of transplantation with induction therapy, but further studies are warranted.
Compared to the established role of MMF as secondary agent in maintenance regimen, the role of PSI/mTOR inhibitor in the maintenance regimen is not yet established due to side effects and lack of long term safety and efficacy data. At present, PSI/mTOR inhibitor combined with low dose CNI regimen is adopted mainly in patients with established CAV and malignancies.
Glucocorticoid is the standard regimen for induction, maintenance and anti-rejection therapy, although no appropriate randomized controlled trials have been done so far. However, because of its deleterious side effects, it is usually tapered to low dose or withdrawn within the first year. Moreover, there is increasing tendency for withdrawing the drug earlier. Only 63% of patients were using prednisone at 1 year post-transplant between 2006 and 2007, only 54% were maintaining the drug at 5 year post-transplant who were transplanted between 1982 to 2006 according to the ISHLT [42].There are 2 approaches to steroid withdrawal, early withdrawal which tapers within the first month and late withdrawal which tapers during 6 to 12 month. Steroid and CNI minimization strategies are especially important in pediatric transplantation to minimize the side effects.
There are no gold standard in immunosuppressive protocols and each drug or combinations of drugs have different impact on transplantation survival. So it is important to individualize the choice of immunosuppressive agents depending on the risk of rejection and comorbidities of the patients.
Acute cellular rejection (ACR) is a T cell mediated inflammatory response to the graft with infiltration of lymphocytes and macrophages. ACR can occur at any time after transplantation but most frequently during the first 3-6 months and is related to lapse in immunosuppressive therapy. Nearly 40% of adult heart transplant patients have one or more acute rejection episodes to some degree in the first month, about 60% experience rejection in 6 months and only one third remain free of rejection at 1 year after transplantation. Risk factors are younger age of recipient, female gender of donor and recipient, higher HLA mismatch, and black recipient. ACR is a risk factor for CAV and mortality.
Diagnosis is made by EMB with the grading system proposed by Billingham initially in 1990 which was revised in 2004 (Table 3)[49]. Treatment is guided by clinical symptom, graft dysfunction and severity of ISHLT criteria on EMB. In asymptomatic patients with low grade ISHLT grade without graft dysfunction, augmentation of current immunosuppressive therapy or a course of steroid bolus can be given. In patients with graft dysfunction, intravenous pulse steroids are usually indicated to decrease the myocardial injury along with or without ATG (Table 5).
Antibody mediated rejection (AMR) or humoral rejection or vascular rejection is characterized by features of vasculitis affecting capillaries such as capillary endothelial change, macrophage and neutrophil infiltration, interstitial edema and perivascular accumulation of antibodies and complements. It is perceived as a clinicoimmunopathologic spectrum from latent phase of circulating Ab only, to silent phase of circulating Ab with C4d deposition in graft, to subclinical phase with circulating Ab, C4d deposition and histologic change in graft, to symptomatic phase with clinical manifestation.
It is more commonly associated with hemodynamic compromise with graft dysfunction, increased graft loss, CAV and high mortality. It often presents in the first month after transplantation accompanied by increase of DSA. About 15% seem to develop AMR in adult heart transplant patients. Risk factors are female gender, high pretransplant PRA, positive crossmatch, CMV seropositivity and prior VAD or retransplantation.
AMR was first described in 1987 as a type of rejection characterized by arteriolar vasculitis with poor outcome. Since then, diagnosis and treatment remained unsolved because of unstandardized diagnostic criteria and current immunosuppressive regimens were largely targeting T cells. However, consensus were driven at the 2010 ISHLT meeting and nowadays diagnosis of AMR is based on the 2010 report (Table 4) [50,51]. The new criteria is based on EMB with histologic findings of AMR of endothelial activation, intravascular macrophages, capillary destruction and immunologic finding of perivascular precipitation of antibodies and complement (C4d, C3d, CD68 for macrophage etc.) without clinical diagnosis and DSA. Although not included in diagnostic criteria, presence of DSA inclines to aggressive treatment. Similar to ACR, decision for treatment is based on the clinical finding of symptom, graft function, EMB grade and/or presence of DSA.
Though it is still controversial, asymptomatic incidental finding of AMR (pAMR1 or pAMR2 without DSA) on surveillance protocol do not warrant treatment in general if cardiac function is preserved. There are reports that they are prone to increased mortality and CAV, however, it is unclear if interrogation would affect the clinical outcome.
Symptomatic or asymptomatic AMR with graft dysfunction needs aggressive treatment with steroid pulse, IVIg, rituximab or bortezomib targeting to block antibody production by B cell and plasma cell. In the setting of heart failure or cardiogenic shock, since ACR and AMR are merged together, patients require a comprehensive approach with high dose steroid pulse, ATG, PP, IVIg with or without IABP or ECMO for hemodynamic support, although mortality remains very high in these patients (Table 5).
There is no evidence based guidelines for the treatment of acute rejection in heart transplantation because of the lack of randomized controlled trials, thus, surveillance monitoring and treatment protocols for rejection remain largely empiric and vary among different transplant centers.
CAV is an important chronic complication limiting long term graft survival which is mediated by predominantly immunologic mechanism, but shares major risk factors with conventional atherosclerosis. One third of patients develop CAV within 5 years and a half within 10 years. IVUS can increase sensitivity in addition to standard diagnostic coronary angiography. PSI/mTORinhibitors (sirolimus and everolimus) have been shown to decrease the incidence of CAV compared to standard regimen with CNI. Oral statin therapy seems to decrease the incidence of CAV, therefore it should be prescribed in every cardiac transplant patient with consideration of drug interactions. Percutaneous coronary intervention (PCI) can relieve symptoms in favorable coronary anatomy but often end up listing for retransplantation with poor prognosis.
Heart transplantation is the final option that can offer long term survival benefit in refractory end stage heart failure patients in this era. Advancement of immunology, sensitive and specific immune monitoring technology, refined and targeted immunosuppressive drugs, advancement in the management of complications and mechanical assist devices for the rescue therapy contributed to these achievements. Appropriate patient selection and individualized immunosuppression is the key to the successful outcome in heart transplantation.
Donor shortage is the major limitation of heart transplantation. However, there are ongoing efforts to improve donor heart allocation and graft preservation. Moreover, technical advancement enabled LVAD to be implanted in replacement of for destination therapy in transplantation ineligible patients and offered LVAD as a bridge to transplantation for the morbid candidates in the waiting list. It has improved survival benefit compared to medical treatment alone but device associated complications and cost limits its widespread application. Further refinement in technology of mechanical assist device is necessary to overcome the barriers in the future.
There has been steady increase in heart transplantations in Korea but candidates in waiting lists are increasing even faster. The short and long term survival is comparable to the survival of ISHLT and widespread application of mechanical assist device in heart failure is expected to happen in the very near future.
Figures and Tables
 | Fig. 2(A) Kaplan-Meier survival curve of heart transplantation from the 2013 ISHLT report [1], (B) Survival curve in Korea during 1992-2012 (from presentation at APCHF 2013), (C) Survival curve of continuous flow LVADs and BiVADs from 5th INTERMACS report. |
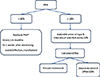 | Fig. 3Proposed strategy of management of the sensitized patients [51]. *May vary among transplantation centers. |
Table 2
Various long term and short term mechanical circulatory support devices in commercial use or ongoing clinical studies [21]

Table 4

Histologic finding includes endothelial activation with intravascular macrophages and capillary destruction. Immunologic finding includes complement and anti HLA Ab deposition. Severe AMR denotes histopathologic findings of interstitial hemorrhage, capillary fragmentation, mixed inflammatory infiltrates, endothelial pyknosis and/or karyorrhexis and marked edema.
ACKNOWLEDGMENT
We thank to Dr. Eun Seok Jeon for providing the statistical data of heart transplantations in Korea.
References
1. Lund LH, Edwards LB, Kucheryavaya AY, Dipchand AI, Benden C, Christie JD, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Official Adult Heart Transplant Report--2013; focus theme: age. J Heart Lung Transplant. 2013; 32:951–964.

2. Kinkhabwala MP, Mancini D. Patient selection for cardiac transplant in 2012. Expert Rev Cardiovasc Ther. 2013; 11:179–191.

3. Colvin-Adams M, Valapour M, Hertz M, Heubner B, Paulson K, Dhungel V, et al. Lung and heart allocation in the United States. Am J Transplant. 2012; 12:3213–3234.

4. The Korean Liver Cancer Study Group. National Cancer Center. Practice guidelines for management of hepatocellular carcinoma [Internet]. Seoul (KR): Korean Medical Guideline Information Center;2014. cited 2014 Jun 1. Available from: http://www.guideline.or.kr/guideline/guide/contents.php?number=41&F_sid=908/.
5. The Organ Procurement and Transplantation Network (OPTN) Annual Data Report [Internet]. Washington, DC (USA): U.S Department of health & human services;2014. cited 2014 Jul 1. Available from: http://srtr.transplant.hrsa.gov/annual_reports/2012/Default.aspx/.
6. Jung SH, Kim JJ, Choo SJ, Yun TJ, Chung CH, Lee JW. Long-term mortality in adult orthotopic heart transplant recipients. J Korean Med Sci. 2011; 26:599–603.

7. Oh SI, Oh BH, Rho JR, Kim KB, Kim JJ, Song MG, et al. Results of heart transplantation in Korea. Korean J Med. 2001; 60:228–233.
8. Lee GY, Choi J-O, Jeon ES. Current status of adult heart transplant in Korea: twenty-year experience. J Card Fail. 2014; 20:S103.

9. Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001; 345:1435–1443.

10. Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009; 361:2241–2251.

11. Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007; 357:885–896.

12. Aaronson KD, Slaughter MS, Miller LW, McGee EC, Cotts WG, Acker MA, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012; 125:3191–3200.

13. A Prospective, Randomized, Controlled, Un-blinded, Multi-Center Clinical Trial to Evaluate the HeartWare® Ventricular Assist System (VAS) for Destination Therapy of Advanced Heart Failure [Internet]. United States: A service of the U.S. National Institutes of Health;2014. cited 2014 Jul 17. Available from: http://clinicaltrials.gov/ct2/show/NCT01166347?term=NCT01166347&rank=1/.
14. Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013; 32:141–156.

15. Lietz K, Miller LW. Improved survival of patients with end-stage heart failure listed for heart transplantation: analysis of organ procurement and transplantation network/U.S. United Network of Organ Sharing data, 1990 to 2005. J Am Coll Cardiol. 2007; 50:1282–1290.

16. Patlolla V, Patten RD, Denofrio D, Konstam MA, Krishnamani R. The effect of ventricular assist devices on post-transplant mortality an analysis of the United network for organ sharing thoracic registry. J Am Coll Cardiol. 2009; 53:264–271.
17. Healy AH, Baird BC, Drakos SG, Stehlik J, Selzman CH. Impact of ventricular assist device complications on posttransplant survival: an analysis of the United network of organ sharing database. Ann Thorac Surg. 2013; 95:870–875.

18. John R, Pagani FD, Naka Y, Boyle A, Conte JV, Russell SD, et al. Post-cardiac transplant survival after support with a continuous-flow left ventricular assist device: impact of duration of left ventricular assist device support and other variables. J Thorac Cardiovasc Surg. 2010; 140:174–181.

19. Miller LW, Guglin M. Patient selection for ventricular assist devices: a moving target. J Am Coll Cardiol. 2013; 61:1209–1221.
20. Starling RC, Naka Y, Boyle AJ, Gonzalez-Stawinski G, John R, Jorde U, et al. Results of the post-U.S. Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol. 2011; 57:1890–1898.

22. Park SJ, Milano CA, Tatooles AJ, Rogers JG, Adamson RM, Steidley DE, et al. Outcomes in advanced heart failure patients with left ventricular assist devices for destination therapy. Circ Heart Fail. 2012; 5:241–248.

23. Boyle AJ, Ascheim DD, Russo MJ, Kormos RL, John R, Naka Y, et al. Clinical outcomes for continuous-flow left ventricular assist device patients stratified by pre-operative INTERMACS classification. J Heart Lung Transplant. 2011; 30:402–407.

24. Starling RC, Moazami N, Silvestry SC, Ewald G, Rogers JG, Milano CA, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014; 370:33–40.

25. Kobashigawa JA, Sabad A, Drinkwater D, Cogert GA, Moriguchi JD, Kawata N, et al. Pretransplant panel reactive-antibody screens. Are they truly a marker for poor outcome after cardiac transplantation? Circulation. 1996; 94:Ii294–Ii297.
26. Stehlik J, Edwards LB, Kucheryavaya AY, Aurora P, Christie JD, Kirk R, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult heart transplant report--2010. J Heart Lung Transplant. 2010; 29:1089–1103.

27. Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969; 280:735–739.

28. Cecka JM. Calculated PRA (CPRA): the new measure of sensitization for transplant candidates. Am J Transplant. 2010; 10:26–29.

29. Stehlik J, Islam N, Hurst D, Kfoury AG, Movsesian MA, Fuller A, et al. Utility of virtual crossmatch in sensitized patients awaiting heart transplantation. J Heart Lung Transplant. 2009; 28:1129–1134.

30. Schaffer JM, Singh SK, Reitz BA, Oyer PE, Robbins RC, Mallidi HR. Heart transplant graft survival is improved after a reduction in panel reactive antibody activity. J Thorac Cardiovasc Surg. 2013; 145:555–564. discussion 64-5.

31. Kobashigawa JA, Patel JK, Kittleson MM, Kawano MA, Kiyosaki KK, Davis SN, et al. The long-term outcome of treated sensitized patients who undergo heart transplantation. Clin Transplant. 2011; 25:E61–E67.

32. Schumacher KR, Ramon DS, Kamoun M, Caruthers R, Gajarski RJ. HLA desensitization in pediatric heart transplant candidates: efficacy of rituximab and IVIg. J Heart Lung Transplant. 2012; 31:1041–1042.

33. Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai CH, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008; 359:242–251.

34. Patel JK, Everly MJ, Kittleson M, Kobashigawa JA. Use of bortezomib as adjunctive therapy for densensitization in combined heart and kidney transplantation--a case report. Clin Transpl. 2009; 347–349.
35. Perry DK, Burns JM, Pollinger HS, Amiot BP, Gloor JM, Gores GJ, et al. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant. 2009; 9:201–209.

36. Beniaminovitz A, Itescu S, Lietz K, Donovan M, Burke EM, Groff BD, et al. Prevention of rejection in cardiac transplantation by blockade of the interleukin-2 receptor with a monoclonal antibody. N Engl J Med. 2000; 342:613–619.

37. Mehra MR, Zucker MJ, Wagoner L, Michler R, Boehmer J, Kovarik J, et al. A multicenter, prospective, randomized, double-blind trial of basiliximab in heart transplantation. J Heart Lung Transplant. 2005; 24:1297–1304.

38. Deng MC, Eisen HJ, Mehra MR, Billingham M, Marboe CC, Berry G, et al. Noninvasive discrimination of rejection in cardiac allograft recipients using gene expression profiling. Am J Transplant. 2006; 6:150–160.

39. Pham MX, Teuteberg JJ, Kfoury AG, Starling RC, Deng MC, Cappola TP, et al. Gene-expression profiling for rejection surveillance after cardiac transplantation. N Engl J Med. 2010; 362:1890–1900.

40. Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Desai S, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010; 29:914–956.

41. Grimm M, Rinaldi M, Yonan NA, Arpesella G, Arizon Del, Pulpon LA, et al. Superior prevention of acute rejection by tacrolimus vs. cyclosporine in heart transplant recipients--a large European trial. Am J Transplant. 2006; 6:1387–1397.

42. Taylor DO, Barr ML, Radovancevic B, Renlund DG, Mentzer RM Jr, Smart FW, et al. A randomized, multicenter comparison of tacrolimus and cyclosporine immunosuppressive regimens in cardiac transplantation: decreased hyperlipidemia and hypertension with tacrolimus. J Heart Lung Transplant. 1999; 18:336–345.

43. Eisen HJ, Tuzcu EM, Dorent R, Kobashigawa J, Mancini D, Valantine-von Kaeppler HA, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003; 349:847–858.

44. Kaczmarek I, Ertl B, Schmauss D, Sadoni S, Knez A, Daebritz S, et al. Preventing cardiac allograft vasculopathy: long-term beneficial effects of mycophenolatemofetil. J Heart Lung Transplant. 2006; 25:550–556.

45. Vigano M, Tuzcu M, Benza R, Boissonnat P, Haverich A, Hill J, et al. Prevention of acute rejection and allograft vasculopathy by everolimus in cardiac transplants recipients: a 24-month analysis. J Heart Lung Transplant. 2007; 26:584–592.

46. Eisen HJ, Kobashigawa J, Starling RC, Pauly DF, Kfoury A, Ross H, et al. Everolimus versus mycophenolatemofetil in heart transplantation: a randomized, multicenter trial. Am J Transplant. 2013; 13:1203–1216.

47. Kobashigawa JA, Miller LW, Russell SD, Ewald GA, Zucker MJ, Goldberg LR, et al. Tacrolimus with mycophenolatemofetil (MMF) or sirolimus vs. cyclosporine with MMF in cardiac transplant patients: 1-year report. Am J Transplant. 2006; 6:1377–1386.

48. Potena L, Prestinenzi P, Bianchi IG, Masetti M, Romani P, Magnani G, et al. Cyclosporine lowering with everolimus versus mycophenolatemofetil in heart transplant recipients: long-term follow-up of the SHIRAKISS randomized, prospective study. J Heart Lung Transplant. 2012; 31:565–570.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download