Abstract
Hepatocytes, parenchymal cells of the liver, are specially differentiated cells to perform most of the body metabolisms. Many clinicians are interested in utilizing hepatocytes as cell therapeutics. A great number of investigators have been harvesting hepatocytes using two-step portal vein perfusion method, in which Ca2+-free EDTA-containing buffer and Ca2+-enriched collagenase solution are pumped into liver in sequence. Among various attempts for long-term culture of hepatocytes, collagen gel sandwich configuration is recognized to be the most effective technique. In the biomedical field, hepatocytes have been used in three methods of applications. First is hepatocyte transplantation for the treatment of acute, chronic liver failure and metabolic diseases. Donated livers not suitable for organ transplantation are rare, which is the major human hepatocyte source. This shortage of human hepatocyte source is expected to be resolved by virtue of rapid progressing stem cell technologies. The second application is biological components of bioartificial liver (BAL) system for acute liver failure patients. Due to the lack of functional activity of clinically studied BAL systems and difficulty of establishing a manufacturing system for ready-to-use, additional research activities are stagnated. The third utilization of hepatocytes is in vitro drug screening studies such as drug metabolism, transport, biliary excretion, and toxicity tests. If cell therapeutic treatments using hepatocytes are clinically valuable to some types of liver diseases, the demand for liver transplantation would be significantly diminished.
The liver plays a major role in in vivo metabolisms such as in synthesis of plasma proteins, blood glucose generation, urea formation, secretion of bile, and detoxification. Since most of these hepatic functions are performed by hepatocytes, parenchymal cells of the liver, there has been much attention to their use for cell therapeutic applications and the in vitro studies of liver physiology, drug metabolism, and hepatic toxicology [1]. However, hepatocytes are well known to dedifferentiate after isolation and lose much of their specific functions within two days under conventional culture conditions such as a monolayer on plastic surface [2]. Consequently, there have been many attempts to maintain and improve hepatocyte functions in vitro including manipulation of soluble factors, cell-cell and cell-matrix interactions. Sandwich configuration [3], in which hepatocytes are cultured between two hydrogel layers of collagen, and co-culture [4], in which hepatocytes are cultured together with other nonparenchymal cells, are the most remarkable techniques.
In this paper, we first review hepatocyte isolation and culture techniques to secure a cell source and then examine hepatocyte transplantation (HTx) and bioartificial liver (BAL) studies and describe nonclinical but important uses of human hepatocytes for in vitro pharmacokinetic studies.
Various methods have been employed in attempts to isolate hepatocytes. These include mechanical, chemical, and enzymatic methods as well as a combination of these methods (Fig. 1). However it was very difficult with the earlier mechanical and chemical methods to isolate hepatocytes with sufficient viability [5]. Since collagenase has been used for the enzymatic digestive method, the viability, yield and their functions have improved significantly. In 1967, Howard et al. tried to use collagenase and hyaluronidase. However they did not have high cell yield because of poor digestion made by shaking liver tissue fragments minced in the enzyme solution without perfusion [6]. Berry and Friend obtained markedly improved results by perfusing these enzymes at 37℃ through the portal vein [7]. Since the description of the collagenase perfusion technique was introduced, numerous minor changes have been made so far. The most important modification so called "two step procedure" was originally introduced by Seglen, in which perfusion of the liver with a Ca2+ and enzyme free buffer and followed by a perfusion with a Ca2+-enriched collagenase-containing buffer solution [8]. Today, most investigators have been isolating hepatocytes by means of the two step procedures or that with some modification from various hepatic tissues with good yield and viability. In order to increase the yield and viability of hepatocytes, some investigators perfuse the liver with five-step, EDTA, Ca2+-free, low Ca2+, collagenase and cold washing buffers in sequence, via dual vessel portal vein and hepatic artery [9]. However, this complex perfusion method is also only a modified procedure of the two-step procedure.
The only probable source of human liver tissue for hepatocyte isolation is a discarded organ that was donated for orthotopic liver transplantation (OLT) but not suitable for transplantation due to fatty change or extended period of ischemic condition [10]. The liver with a large mass such as an adult human liver can be used for hepatocyte isolation when equipped with the high flow-capacity perfusion device up to 1-2 L/min. With the improvement of OLT technology, donated livers with marginal conditions were frequently transplanted to patients. So, the environment of securing the human liver for hepatocytes preparation is becoming poor. Recently, non-heart beating donor (NHBD) livers have been suggested as an alternative liver source for hepatocytes [11]. Hypothermic oxygenated perfusion pretreatment of NHBD liver and N-acetylcysteine treatment of severely steatotic liver demonstrate a beneficial effect in reducing reperfusion and ischemic injuries [12,13]. In the US and EU countries human hepatocytes for in vitro studies such as drug metabolism studies have been obtained from transplantation-unsuitable donated livers. Considering that such cases are very rare in Korea, normal liver tissue obtained from liver resection surgeries is a reasonable liver tissue source for human hepatocyte isolation [14]. Liver perfusion procedures for isolating hepatocytes from resected liver tissues are the same as those of whole liver perfusion. Because there may be many openings of blood vessels on the cut surface, multiple perfusion cannulae and ligation of bypassing vessels are beneficial for maximal perfusion-of the liver fragments which increase yield and viability of the hepatocytes. Warm ischemia of liver tissues before starting the hepatocyte isolation procedure is a major factor to determine hepatocyte viability and yield. More than one hour of warm ischemic time is detrimental for achieving intact hepatocytes with high yields.
Hepatocytes have a unique epithelial cell polarity and intercellular structures including bile canaliculi, tight and gap junctions, and the cells also possess high regeneration capability. But they lose these specific properties very fast when isolated and cultured in vitro [2]. For this reason, hepatocytes have been known as one of the most demanding cells to maintain or proliferate in vitro. Among various culture techniques, spheroid culture, collagen sandwich culture and co-culture methods will be discussed in detail here.
Several investigators have found that freshly isolated primary hepatocytes can form tightly attached multicellular aggregates, so called spheroids [15]. This specialized spherical structure has been observed to exhibit improved hepatic functions and extended maintenance of the unique morphologies compared to the cells that were cultivated as a monolayer [15]. Usually the spheroids were formed under conditions in which attachment of cells to substratum are prevented [16]. Suspension culture and seeding on a less-adherent surface [16] are the most common methods to form hepatocyte spheroids. This culture method still has been improved to control culture conditions precisely such as size and inclusion of non-parenchymal cells with the help of a bio-MEMS (Micro-electro-mechanical systems) technology [17].
The space of Disse was not an empty space, as seen by conventional electron microscopy, but contained a unique low-density extracellular matrix (ECM). This matrix includes type I collagen, free, unassociated type IV collagen, and fibronectin. In vivo hepatocytes are enclosed within the ECM of the space of Disse. For this reason the effects of various extracellular matrix components on cultured hepatocytes have been investigated. When hepatocytes were inoculated on collagen or fibronectin-coated plate initial attachment was increased and the state was maintained longer than those plated on conventional culture surfaces [18]. However, such typical extracellular matrix components do not prevent transformation of hepatocytes in culture and even facilitate the spread of the cells which accelerate change of the gene expressions. It has been verified that a round cell shape, unflattened morphology is favorable to maintain the differentiated state with hepatocytes for longer culture period [19]. For example, hepatocytes cultured on laminin-rich collagen gel, called matrigel represent more round cell shapes and significantly enhanced liver specific functions for a longer period [19].
As an effort to mimic the micro-environment of hepatocytes in the liver in which the cells exist as a cell plate surrounded by scant ECM in Disse space, Dunn et al. [3] cultured hepatocytes between collagen hydrogel layers. In this sandwich configuration, hepatocytes maintain high level of hepatic functions such as albumin secretion and cytochrome P-450 activities, etc. This simple but powerful method is now widely used for drug metabolism and toxicology studies [20]. In addition, membrane-supported collagen sandwich and chemically modified collagen gels were also shown to be appropriate for culture of hepatocytes. Fig. 2 shows the simplified progress of hepatocyte culture configurations using ECM components. Sandwich culture configuration is also being applied to the bioreactor for the BAL system [21].
Hepatocytes actively interact with several sinusoidal cells such as sinusoidal endothelial cells, stellate cells and Kupffer cells and have direct contacts with the bile duct epithelial cells at the canals of the Hering region. To reflect this in vivo environment to culture systems, various liver non-parenchymal cells, such as epithelial cells, and sinusoidal endothelial cells, and fibroblastic cells were co-cultured with hepatocytes [22]. The first attempts to co-culture hepatocytes with sinusoidal cells or human fibroblasts showed limited and transient enhancements of liver functions. Breakthrough in the culture technique was made by using untransformed epithelial cells derived from neonate rat liver [4]. When co-cultured with these epithelial liver cells, hepatocytes retain high functional capacities, including production of plasma proteins and phase I and II drug metabolisms. This co-culture technique has contributed to provide a platform toward hepatic tissue engineering in vitro [23].
Unlike the case with other cell cultures, fetal bovine serum (FBS) is not appropriate to hepatocyte culture for some reasons. FBS is sometimes used for short term culture because it is helpful for early attachment of hepatocytes, but it impedes bile secretion of hepatocytes by inhibiting formation of bile canaliculi [24], and always induce overgrowth of fibroblasts. Thus, serum-free medium is preferred and medium additives such as EGF, insulin, glucocorticoid hormone and glucagon are needed to maintain the differentiated state of hepatocytes [25].
The first experiment to treat liver failure by hepatocyte transplantation (HTx) was performed in 1976 in the hyperbilirubinemic Gunn rat [26]. Where after, non-clinical studies have been attempted to assess the potential of the cell transplantation strategies in several animal models with congenital metabolic diseases including the Crigler-Najjar syndrome type 1 rat, ornithine transcarbamylase deficiency mice, Wilson's disease rat, Nagase analbuminemic rats, etc. [27]. The first human hepatocyte transplantation was attempted by Mito et al. in 1993. Patients with chronic liver disease received infusion of autologous hepatocytes into the spleen [28]. HTx has several advantages over OLT. Because the procedure is less invasive than those of OLT surgery, the risk associated with the treatment can be reduced significantly. Furthermore, the numbers of hepatocytes that can be obtained from a single donor liver are sufficient for several recipients. Timing is less critical for HTx than OLT because hepatocytes can be cryopreserv-ed and supplied after thawing when needed. In addition, there is the potential of genetic modification of hepatocytes in vitro that may upregulate specific functions. If we are considering autologous transplantation of ex vivo genetically modulated hepatocytes, it gives an opportunity to avoid costly immunosuppression that cause harmful adverse events [29]. HTx has been proposed as a treatment for acute and chronic liver disease, as well as metabolic disease of the liver [30]. Hence, HTx has been used for transient liver support of patients in end-stage liver failure awaiting OLT, as a method to support liver function and give the opportunity of the patient's liver to regenerate on its own in cases of fulminant hepatic failure, and in same concepts with gene therapy, as a cell therapy for the treatment of congenital metabolic diseases.
There are a number of centers worldwide that have reported their experience of clinical HTx. None of these studies demonstrated a complete cure; however, many of them reported improvement in phenotype from severe to moderate or mild. The indications included liver-based metabolic disorders such as ornithine transcarbamylase deficiency, arginine succinyllyase deficiency, citrullinemia, Crigler-Najjar syndrome type I, glycogen storage disease 1a and 1b, Refsum's disease, factor VII deficiency (FVII), progressive familial intrahepatic cholestasis type II, and hypercholesterolemia; acute liver failure, and a few reports on chronic liver disease [31]. The ultimate goal of HTx in hepatic failure is to replace the function for lifelong periods. However, only 20 months is the longest period that the transplanted cells were able to be maintained in the recipient liver even though some acute liver failure cases fully recovered their liver function [32].
HTx is not yet an established therapy in that there are many challenges to be solved such as isolation and preservation of human hepatocytes, selection of target indications, cell administration route and times, cell function monitoring, immunosuppression strategy, graft and repopulation enhancement, etc. In this review, only cell delivery practice and cell engraft improvement efforts will be mentioned.
Animal models for HTx suggested that there are several possible sites suitable for hepatocyte transplantation, however, in humans, the preferred route for metabolic indications is intraportal. Intraportal injection of hepatocytes causes portal hypertension and cell thrombi however, these adverse events can be minimized by adding anticoagulant to cell solution and limiting the cell numbers transplanted, infusion flow rate and cell density in transplantation protocols according to the weight of recipients. In order to increase the total number of implanted hepatocytes, repeated transplantation is inevitable. In this case long-term portal vein access should be taken into account in the HTx procedures. When recipients have chronic liver disease with severe fibrotic changes that are considered as harsh conditions for hepatocytes to engraft, the spleen is a good alternative route for hepatocyte transplantation. The peritoneal cavity has been also used for the transplantation of relatively large cell mass in case of acute liver failure [33].
Various approaches have been used to improve cell engraftment in the preclinical model. First, isolated hepatocytes were shown to express the tissue factors that have procoagulant activity. It demonstrated that N-acetylcysteine inhibits this tissue factor-dependent activation of coagulation. Second, because the sinusoid is smaller than hepatocytes, vasodilators such as nitroglycerine have been shown to increase transplanted cell entry into liver sinusoids. Third, transplanted cells were cleared rapidly by the phagocytic cellular immune system such as Kupffer cells and then the surviving allogeneic cells were recognized by the acquired immune system such as CD4+ and CD8+ T cells. Additional studies are needed for better understanding of the immune mechanisms associated with HTx and to improve the appropriate immunosuppressive protocols. Fourth, increased cell engraftment based on portal embolization or irradiation of the liver was shown in metabolic disease models [34,35]. Ongoing clinical studies registered at international database ClinicalTrials.gov using hepatocytes for treatment of liver failure is given in Table 1. Although it is not registered in the clinical study database, clinical study on hepatocyte transplantation for the treatment of inborn metabolism disorder is ongoing only in Samsung Seoul Hospital, Korea.
So far, more than nine types of BAL systems have been clinically investigated, and their feasibility as a use of liver support system was partially verified. None of the BAL systems received marketing approval that needs proof of its safety and survival gains [36]. As a brief review, the characteristics of eight representative BAL systems including upgraded versions of ELAD and HepatAssistsystems are listed in Table 2.
The Extracorporeal Liver Assist Device (ELAD) is the only BAL system that uses the human hepatoma cell line C3A, which is derived from the HepG2 cell line [37]. C3A cells express normal hepatic metabolisms such as urea formation, gluconeogenesis and cytochrome P-450 activities, and secrete albumin and α-fetoprotein. The cells are proliferated in the extracapillary space of hollow fiber cartridges and blood flows into the lumen of hollow fibers. A portion of the patient's plasma is permeated across the 70 kD molecular weight cut-off membrane and is in direct contact with the C3A cells. In a pilot controlled trial on 24 acute liver failure patients, there was no significant difference in survival rate, renal function, and biochemical parameters between the ELAD-treated patients and the controls [37]. Afterward, Phase III clinical trial is ongoing using a performance-improved bioreactor [38].
The HepatAssist system developed by Demetriou and coworkers was the only system examined in the largest controlled phase III clinical trial [39]. In this hollow fiber bioreactor, cryopreserved and thawed porcine hepatocytes are mixed with microcarriers and then placed in the extracapillary space [40]. They particularly use 0.2 µm-pore hollow fiber membranes that had been used for the plasma separation device. Pre-separated patient's plasma passes through a toxin-absorption column and then flows into the lumen of the hollow fibers. The first company that had developed the BAL system was acquired by another company and the company is preparing additional clinical trials with an improved system using pig fetal liver-derived cell line (PICM-19H) [41].
The Liver Support System (LSS) developed by Gerlach and coworkers, employed a specially designed hollow fiber bioreactor in which four different hollow fiber membranes were woven as a 3D lattice. These four types of hollow fibers are utilized as an oxygen, nutrient supply and plasma inflow and outflow of the bioreactor. Extracapillary space formed by the woven hollow fibers was inoculated with mixture of hepatocytes and nonparenchymal cells. Then the cells aggregated as small tissue-like organoids consisting of hepatocytes and nonparenchymal cells such as sinusoidal endothelial cells. The LSS is the only system that uses primary human hepatocytes isolated from discarded donor livers as well as porcine hepatocytes [42]. In a phase I clinical study using human hepatocytes, the LSS was combined with a single-pass albumin dialysis machine (MARS), called the modular extracorporeal liver support (MELS) [42].
The radial flow bioreactor (RFB) BAL system was developed in the University of Ferrara, Italy [43]. In this system, the patients' plasma perfused from the center to the periphery of the bioreactor, namely radial flow. Oxygen consumption rate was measured to evaluate the cellular activities of the bioreactor, thereafter, the consumption rate decreases during the treatment of patients, which represents necrosis of the hepatocytes.
The Academic Medical Center (AMC) BAL system developed by Chamuleau and coworkers in the Universityof Amsterdam, Netherlands, consists of a nonwoven hydrophilic polyester porous sheet-type matrix for cell attachment and hollow fibers for oxygen supply that also act as a spacer of the sheets [44]. The porous 4 mm thickness sheet is spirally wound with longitudinal oxygen supplying hollow fibers. In the AMC-BAL bioreactor, small hepatocyte aggregates located in pores of the matrix directly contact with the patient's plasma for maximal mass transfer. Configuration of the AMC-BAL reactor has been upgraded to enhance liver functions [45]. According to the company's website, HepaRG cells and liver cancer-derived precursor cell lines are considered as a cell source of the BAL system in next clinical studies.
LifeLiver developed relatively later than any other BAL systems and it was focused on efficacy of the bioreactor according to the latest trend of BAL systems, and is on phase I/IIa clinical trial in Samsung Seoul Hospital, Korea. Bioreactor configuration of LifeLiver is a fixed and packed-bed module containing hepatocyte spheroid-entrapped Ca-alginate beads [46]. While the alginate beads provide an immune barrier against patient's plasma, it shows sufficient mass transfer efficiency required to maintain liver functions.
Many researchers continued to improve hepatocyte culture me-thod and apply it to the new BAL system. Stem cell-derived hepatocytes which have been studied as an alternative source of primary hepatocytes of the BAL system have not yet shown proper hepatic functions [47]. Seeing that most of cell sources of the recent BAL system in clinical trials are almost hepatoma-derived cell lines, the BAL system using stem cell-derived hepatocytes seems a long way to go. Hepatocytes are one of the most difficult cells to preserve in hypothermic and cryopreserved condition for a long time. For this reason, it is difficult to maintain BAL systems in ready-to-use state for the emergent clinical situation.
Therefore, until a long-term preservation method of hepatocytes is established, centralized regional medical centers that are specialized in BAL application are required to increase the treatment incidence of acute liver failure, up to around two or three cases per a week. At this treatment incidence, it is possible to maintain ready-to-use BAL systems all the time for urgent clinical application.
Whereas cell therapeutic applications of cultured hepatocytes are not widely used yet clinically, cultured hepatocytes already occupies a very important position in the drug screening process [20]. At present, isolated hepatocytes were regarded as one of the most appropriate and practical models for studying drug metabolism and transporter and drug-interactions. Microsome of liver tissues has been used as a preliminary screening model for early drug candidates. But, microsome-based analysis is being replaced or complemented with human hepatocyte-based analysis [48]. This trend is due to several evidences that primary hepatocytes would be a better model to estimate in vivo metabolic profile qualitatively [49]. The U. S. Food and Drug Administration (FDA) recommended the human primary hepatocyte as a standard model for drug metabolism evaluation and published guidance for the use of human hepatocytes. As mentioned before, sandwich culture configuration has been recommended as a good choice [20]. In addition to drug metabolism studies, cultured hepatocytes also provide a useful in vitro tool for studying fates of hepatitis virus B and C and evaluate therapeutic effects of antiviral drug candidates [50].
The importance and potential of hepatocytes in the treatment of liver diseases already have been confirmed by many clinical studies. Cultured hepatocytes also make contribution to drug screening and the study of viral hepatic diseases. Rapid progressing stem cell technologies can lead to sufficient supply of functional human hepatocytes, and then, the current OLT-dependent technology for liver failure patients will largely change to regenerative treatment by using cell therapeutics.
Figures and Tables
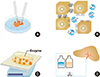 | Fig. 1Brief history of hepatocyte isolation techniques. (A) mechanical dissociation, (B) Ca2+ chelating, (C) enzyme digestion of tissue fragments, (D) two-step enzyme perfusion via portal vein. |
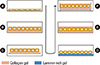 | Fig. 2Schematic diagram of culture configurations of hepatocytes using ECM components. Arrow roughly indicates progress direction of culture techniques. (A) dry coated with collagen or fibronectin, (B) hydrogel of collagen, (C) floating collagen gel, (D) laminin-rich gel (Matrigel), (E) sandwiched by two layers of collagen gel, (F) membrane-supported sandwich culture. |
References
1. Puviani AC, Ottolenghi C, Tassinari B, Pazzi P, Morsiani E. An update on high-yield hepatocyte isolation methods and on the potential clinical use of isolated liver cells. Comp Biochem Physiol A Mol Integr Physiol. 1998; 121:99–109.

2. Reid LM, Jefferson DM. Culturing hepatocytes and other differentiated cells. Hepatology. 1984; 4:548–559.

3. Dunn JC, Tompkins RG, Yarmush ML. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991; 7:237–245.

4. Clement B, Guguen-Guillouzo C, Campion JP, Glaise D, Bourel M, Guillouzo A. Long-term co-cultures of adult human hepatocytes with rat liver epithelial cells: modulation of albumin secretion and accumulation of extracellular material. Hepatology. 1984; 4:373–380.

5. Wang SR, Renaud G, Infante J, Catala D, Infante R. Isolation of rat hepatocytes with EDTA and their metabolic function in primary culture. In Vitro Cell Dev Biol. 1985; 21:526–530.

6. Howard RB, Christensen AK, Gibbs FA, Pesch LA. The enzymatic preparation of isolated intact parenchymal cells from rat liver. J Cell Biol. 1967; 35:675–684.

7. Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969; 43:506–520.
8. Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976; 13:29–83.
9. Gerlach J, Brombacher J, Smith M, Neuhaus P. High yield hepatocyte isolation from pig livers for investigation of hybrid liver support systems: influence of collagenase concentration and body weight. J Surg Res. 1996; 62:85–89.

10. Gerlach JC, Mutig K, Sauer IM, Schrade P, Efimova E, Mieder T, et al. Use of primary human liver cells originating from discarded grafts in a bioreactor for liver support therapy and the prospects of culturing adult liver stem cells in bioreactors: a morphologic study. Transplantation. 2003; 76:781–786.

11. Hughes RD, Mitry RR, Dhawan A, Lehec SC, Girlanda R, Rela M, et al. Isolation of hepatocytes from livers from non-heart-beating donors for cell transplantation. Liver Transpl. 2006; 12:713–717.

12. Schlegel A, Rougemont O, Graf R, Clavien PA, Dutkowski P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J Hepatol. 2013; 58:278–286.

13. Sagias FG, Mitry RR, Hughes RD, Lehec SC, Patel AG, Rela M, et al. N-acetylcysteine improves the viability of human hepatocytes isolated from severely steatotic donor liver tissue. Cell Transplant. 2010; 19:1487–1492.

14. Vondran FW, Katenz E, Schwartlander R, Morgul MH, Raschzok N, Gong X, et al. Isolation of primary human hepatocytes after partial hepatectomy: criteria for identification of the most promising liver specimen. Artif Organs. 2008; 32:205–213.

15. Li AP, Colburn SM, Beck DJ. A simplified method for the culturing of primary adult rat and human hepatocytes as multicellular spheroids. In Vitro Cell Dev Biol. 1992; 28A:673–677.

16. Koide N, Sakaguchi K, Koide Y, Asano K, Kawaguchi M, Matsushima H, et al. Formation of multicellular spheroids composed of adult rat hepatocytes in dishes with positively charged surfaces and under other nonadherent environments. Exp Cell Res. 1990; 186:227–235.

17. Lee SA, No da Y, Kang E, Ju J, Kim DS, Lee SH. Spheroid-based three-dimensional liver-on-a-chip to investigate hepatocyte-hepatic stellate cell interactions and flow effects. Lab Chip. 2013; 13:3529–3537.

18. Bissell DM. Primary hepatocyte culture: substratum requirements and production of matrix components. Fed Proc. 1981; 40:2469–2473.
19. Bissell DM, Arenson DM, Maher JJ, Roll FJ. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. J Clin Invest. 1987; 79:801–812.

20. De Bruyn T, Chatterjee S, Fattah S, Keemink J, Nicolai J, Augustijns P, et al. Sandwich-cultured hepatocytes: utility for in vitro exploration of hepatobiliary drug disposition and drug-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2013; 9:589–616.

21. De Bartolo L, Jarosch-Von Schweder G, Haverich A, Bader A. A novel full-scale flat membrane bioreactor utilizing porcine hepatocytes: cell viability and tissue-specific functions. Biotechnol Prog. 2000; 16:102–108.

22. Morin O, Normand C. Long-term maintenance of hepatocyte functional activity in co-culture: requirements for sinusoidal endothelial cells and dexamethasone. J Cell Physiol. 1986; 129:103–110.

23. Acikgoz A, Giri S, Cho MG, Bader A. Morphological and functional analysis of hepatocyte spheroids generated on poly-HEMA-treated surfaces under the influence of fetal calf serum and nonparenchymal cells. Biomolecules. 2013; 3:242–269.

24. Terry TL, Gallin WJ. Effects of fetal calf serum and disruption of cadherin function on the formation of bile canaliculi between hepatocytes. Exp Cell Res. 1994; 214:642–653.

25. Parzefall W, Erber E, Kainzbauer E, Schulte-Hermann R. Effects of insulin, glucagon and triiodothyronine on DNA synthesis in rat hepatocyte primary cultures induced by liver tumour promoters and EGF. Toxicol In Vitro. 1996; 10:183–193.

26. Matas AJ, Sutherland DE, Steffes MW, Mauer SM, Sowe A, Simmons RL, et al. Hepatocellular transplantation for metabolic deficiencies: decrease of plasms bilirubin in Gunn rats. Science. 1976; 192:892–894.

27. Fitzpatrick E, Mitry RR, Dhawan A. Human hepatocyte transplantation: state of the art. J Intern Med. 2009; 266:339–357.

28. Mito M, Kusano M, Kawaura Y. Hepatocyte transplantation in man. Transplant Proc. 1992; 24:3052–3053.

29. Grossman M, Rader DJ, Muller DW, Kolansky DM, Kozarsky K, Clark BJ 3rd, et al. A pilot study of ex vivo gene therapy for homozygous familial hypercholesterolaemia. Nat Med. 1995; 1:1148–1154.

30. Jorns C, Ellis EC, Nowak G, Fischler B, Nemeth A, Strom SC, et al. Hepatocyte transplantation for inherited metabolic diseases of the liver. J Intern Med. 2012; 272:201–223.

32. Filippi C, Dhawan A. Current status of human hepatocyte transplantation and its potential for Wilson's disease. Ann N Y Acad Sci. 2014; 1315:50–55.

33. Dhawan A, Puppi J, Hughes RD, Mitry RR. Human hepatocyte transplantation: current experience and future challenges. Nat Rev Gastroenterol Hepatol. 2010; 7:288–298.

34. Puppi J, Strom SC, Hughes RD, Bansal S, Castell JV, Dagher I, et al. Improving the techniques for human hepatocyte transplantation: report from a consensus meeting in London. Cell Transplant. 2012; 21:1–10.

35. Hughes RD, Mitry RR, Dhawan A. Current status of hepatocyte transplantation. Transplantation. 2012; 93:342–347.

36. van de Kerkhove MP, Hoekstra R, Chamuleau RA, van Gulik TM. Clinical application of bioartificial liver support systems. Ann Surg. 2004; 240:216–230.

37. Ellis AJ, Hughes RD, Wendon JA, Dunne J, Langley PG, Kelly JH, et al. Pilot-controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology. 1996; 24:1446–1451.

38. Millis JM, Cronin DC, Johnson R, Conjeevaram H, Conlin C, Trevino S, et al. Initial experience with the modified extracorporeal liver-assist device for patients with fulminant hepatic failure: system modifications and clinical impact. Transplantation. 2002; 74:1735–1746.

39. Demetriou AA, Brown RS Jr, Busuttil RW, Fair J, McGuire BM, Rosenthal P, et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004; 239:660–667. discussion 7-70.

40. Demetriou AA, Rozga J, Podesta L, Lepage E, Morsiani E, Moscioni AD, et al. Early clinical experience with a hybrid bioartificial liver. Scand J Gastroenterol Suppl. 1995; 208:111–117.

41. Rozga J. Liver support technology--an update. Xenotransplantation. 2006; 13:380–389.
42. Sauer IM, Zeilinger K, Obermayer N, Pless G, Grunwald A, Pascher A, et al. Primary human liver cells as source for modular extracorporeal liver support--a preliminary report. Int J Artif Organs. 2002; 25:1001–1005.

43. Morsiani E, Pazzi P, Puviani AC, Brogli M, Valieri L, Gorini P, et al. Early experiences with a porcine hepatocyte-based bioartificial liver in acute hepatic failure patients. Int J Artif Organs. 2002; 25:192–202.

44. van de Kerkhove MP, Di Florio E, Scuderi V, Mancini A, Belli A, Bracco A, et al. Phase I clinical trial with the AMC-bioartificial liver. Int J Artif Organs. 2002; 25:950–959.

45. van de Kerkhove MP, Poyck PP, van Wijk AC, Galavotti D, Hoekstra R, van Gulik TM, et al. Assessment and improvement of liver specific function of the AMC-bioartificial liver. Int J Artif Organs. 2005; 28:617–630.

46. Park JK, Lee DH. Bioartificial liver systems: current status and future perspective. J Biosci Bioeng. 2005; 99:311–319.

47. Pan XP, Li LJ. Advances in cell sources of hepatocytes for bioartificial liver. Hepatobiliary Pancreat Dis Int. 2012; 11:594–605.

48. Hewitt NJ, Lechon MJ, Houston JB, Hallifax D, Brown HS, Maurel P, et al. Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab Rev. 2007; 39:159–234.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download