Abstract
Over the last decades, piles of data have been accumulated to understand the olfactory sensation in every aspect, ranging from the intracellular signaling to cognitive perception. This review focuses on the ion conduction through multiple ion channels expressed in olfactory sensory neurons (OSNs) to describe how odorant binding to olfactory receptors is transduced into an electrical signal. Olfactory signal transduction and the generation of the depolarizing receptor current occur in the cilia, where the unique extraciliary environment of the nasal mucosa assists in the neuronal activation. Upon contacting with odorants, OSNs dissociate G protein-coupled receptors, initiating a signal transduction pathway that leads to firing of action potential. This signaling pathway has a unique, two step organization: a cAMP-gated Ca2+ (CNG) channel and a Ca2+-activated Cl- channel (CACC), both of which contribute to signal amplification. This transduction mechanism requires an outward-directed driving force of Cl- established by active accumulation of Cl- within the lumen of the sensory cilia. To permit Cl- accumulation, OSNs avoid the expression of the 'Chloride Sensor: WNK3', that functions as the main Cl- exclusion co-transporter in neurons of the central nervous system (CNS). Cl- accumulation provides OSNs with the driving force for the depolarization, increasing the excitatory response magnitude. This is an interesting adaptation because of the fact that the olfactory cilia reside in the mucus, outside the body, where the concentrations of ions are not as well regulated as they are in normal interstitial compartments.
The 'sweet smell of success' greeted Richard Axel and Linda B. Buck who were awarded the 2004 Nobel Prize in Physiology for their outstanding discovery of olfactory receptor proteins that led to a better understanding of the olfactory pathway. The ability to detect and respond in an adaptive manner to chemical signals serves as the primary window to the sensory world. In Humans, the sense of smell serves mostly aesthetic functions but in many other animal species, olfaction serves as a primal sense of continuance. These animals depend heavily on their sense of smell to detect food, predators or other dangers and to communicate social information. Olfaction also plays a role in mate selection, mother-infant recognition and signaling between members within a group.
The olfactory system and the brain perceive the external environment through their ability to process large amounts of odor signals and translate them into a wide spectrum of responsible behaviors. Volatile chemicals act as stimulants in olfaction binding to receptor proteins expressed on the surface of olfactory receptor neurons (ORNs). ORNs are bipolar neurons with a single dendrite end harboring into the epithelium from which cilia protrude into the nasal mucosa. This ensures that ORNs are in direct contact with the external environment through the ciliary machinery. Within the compact cilia of the olfactory sensory neurons (OSNs) a cascade of enzymatic activity converts the binding of an odorant molecule to the receptor into an electrical signal that can activate OSNs (Fig. 1).
In this review, we will discuss about the elucidation of intracellular Cl- environment and the molecular identity of various components that take part in the signal transduction cascade in OSN. Emphasis is placed on the system's complex and dynamic nature including its unique transduction pathway that initiates a sequence of molecular events leading to sensory transduction, impulse initiation, and the transmission of sensory information to the brain.
The perception of smell begins with the activation of odorant receptors (OR) in the olfactory sensory neurons. The OR family has one of the largest gene pool with around 900 genes in humans and 1,500 in mouse. ORs are thus one of the largest known gene families in the human and mouse genomes, representing 3-5% of all genes. These receptors belong to the superfamily of G protein-coupled receptors and consist of seven hydrophobic membrane-spanning regions. However, they differ in their amino acid sequence in the transmembrane domain III, IV, and V, which suggest that these parts are responsible for the discrimination of odorant species [1]. In the mouse olfactory epithelium, there are about 1,000 genes encoding different types of odorant receptors. Each OSN expresses only one type of odorant receptor gene in its ciliary membrane. A single OR responds to many types of odorants and aditionally, a single type of odorant can stimulate several types of ORs [2]. The binding of the odorant to the receptor changes the conformation of its plasma membrane receptors. The activated receptors catalyzes the exchange of guanosine 5-diphosphate (GDP) for guanosine 5-triphosphate (GTP) on multiple trimeric G proteins causing the dissociation of Gαolf from Gβ and γ [3]. Each Gαolf in turn activates adenyl cyclase type III that converts adenosine triphosphate (ATP) into Cyclic Adenosine Monophosphate (cAMP). cAMP plays an important role in olfaction as a second messenger and me-diates signal transduction for a wide variety of odorants [4,5] (Fig. 1).
Olfactory cilia contains the major components of olfactory signal transduction including high levels of Cyclic Nucleotide-Gated Channels (CNG).These channels play a significant role in mediating chemoelectrical energy conversion in olfactory cilia. The information about the odorant molecules is first transmitted as chemical information and then the CNG channel convert it into an electrical signal by the activation of ion channels across the plasma membrane. These channels consists of four subunits containing two CNGA2, one CNGA4, and one CNGB1b [6]. Each subunit has six transmembrane domains (S1-S6) and a pore region between S5 and S6 domains. A cyclic nucleotide binding site is present near the C-terminal at the cytoplasmic side in each subunit [7]. CNG channels are permeable to monovalent ions, such as Na+ and K+, and also to Ca2+ (Fig. 1).
Historically, the olfactory transduction was regarded as a system that worked similarly to the vertebrate phototransduction in a sense that it involved the activation of G protein-coupled receptors that triggered an electrical response by generating a current through CNG channels. This view was altered when Kleene and Gesteland reported the presence of Ca2+-activated Cl- conductance in olfactory cilia. This Ca2+-activated Cl- current was then found to be part of the odorant-induced current in amphibian [8,9] and mammalian [10] OSNs. This finding provided an explanation for the observation that the olfactory response persisted even in the absence of external Na+ [11,12]. Apparently, olfactory cilia did not contain Ca2+ stores because application of pharmacological agents that caused Ca2+ release from intracellular stores failed to elicit a ciliary Ca2+ transient current [13]. Additionally, Ca2+ signaling in olfactory receptor cells is regulated separately in the cilia and in the cell body, which allows the generation of compartmentalized Ca2+ transient current [14]. This eliminates the cell body as a source of Ca2+ in the cilia and shows that ciliary Ca2+ is regulated exclusively by the CNG channel. Odor stimulation opens the CNG channels and increases the ciliary Ca2+ concentration [15] and this primary Ca2+ signal generates an additional secondary Cl- current that is excitatory under physiological conditions [9,16]. Thus, generation of the receptor response of OSNs is thought to involve the sequential activation of two currents: a primary cationic inward current through CNG channels, which is activated by cAMP and is mainly carried by Na+ and Ca2+, and a secondary Cl- inward current through Ca2+-activated Cl channels (CACC) that is triggered by the influx of Ca2+ (Fig. 1).
Recently, the molecular identity of the Ca2+-activated Cl- channels was discovered independently by three research teams [17,18,19]. Studies on gene expression in the olfactory system support the concept that TMEM16B (ANO2) represents the only Anoctamin at the plasma membrane of OSNs [17]. Ano2 tops a list of genes highly enriched in OSNs over other cells of the Main Olfactory Epithelium [20], and it is the only Anoctamin consistently identified in high amounts in mass spectrometric screens in olfactory membranes [21,22]. No such enrichment was shown for the other Anoc-tamins while Ano1, Ano6, Ano8, and Ano10 are known to be expressed in the MOE [21,23].
A number of studies have shown that calmodulin (CaM) drives regulatory feedback mechanisms that control several key steps of the transduction pathway. During odor detection, CaM is trigger-ed by Ca2+ influx and it reduces ciliary cAMP levels through activation of phosphodiesterase [24] and by inhibition of adenylyl cyclase (AC III), which is a substrate for the CaM-dependent protein kinase CaMK II [25]. Moreover, CaM deactivates the cAMP-gated channel to its ligand and in this way promotes channel closure and rapid adaptation [26]. CaM serves an important link between the two transduction channels by coupling a depolarizing Cl- current to the odor-induced Ca2+ signal. The transduction model illustrates the dual effect of Ca-CaM. The inhibitory effects of Ca-CaM restrict the activity of the cAMP-gated channels to a short time period. The transient Ca2+ influx causes a prolonged elevation of the ciliary Ca2+ concentration and opens multiple Cl- channels, as there are eight times more Cl- channels in the ciliary membrane than cation channels. As a result, the initial small Ca2+ influx triggers a much larger Cl- efflux and generates an excitatory receptor current with Cl- as the main charge carrier. Thus, the presence of this anion-based signal amplification in OSNs [27] points to the evolutionary adaptation of the cells that have to convert small Ca2+ signals into sizeable depolarizations. In this case, Cl- accumulation provides the driving force for a depolarizing Cl- current that is triggered by a Ca2+ signal. CaM plays an important role in this amplification mechanism, as it coordinates both its excitatory and inhi-bitory processes (Fig. 1).
The performance of the olfactory system is highly remarkable because of its odorant detection efficiency and its ability to discriminate between chemically similar odors. However, the olfactory system has to cope with low sensitivity of the receptors that puts serious pressure on the ciliary transduction machinery. Since the production of the second messenger cAMP is limited due to the low efficiency of the initial transduction step, a different process is required in downstream of the transduction cascade. To solve this dilemma sensory cilia of OSN have designed a unique alternative mechanism, that is the accumulation of chloride ions at rest and liberation of a chloride current upon odor detection. This chloride current amplifies the initial neuronal activation (Fig. 1).
Cl- accumulation in ORNs are mediated by the Na+/K+/2Cl- cotransporter, NKCC1 [28,29]. Accordingly, research has shown that genetic knockout of NKCC1 following suppression of Cl- accumulation or Cl- efflux, significantly inhibited the sensory response of ORNs [28,30,31]. Expression of NKCC1 can be found in the ciliary layer of the olfactory epithelium. It seems that other kinases support the normal function of NKCC1 such as SPAK ("STE20/SPS1-related proline/alanine-rich kinase") and OSR1 ("oxidative stress-responsive kinase-1") [32], as well as WNK1 and WNK4 [WNK = "with no lysine (K) kinase"] [33]. NKCC1 serves as a substrate for the two protein kinases SPAK and OSR1 [32]. These kinases are in turn activated by the protein kinases WNK1 and WNK4 [33]. Thus, WNK1 and WNK4 participate indirectly in the control of NKCC1. Recent studies have showed that WNK1 and WNK4 phosphorylate OSR1 and SPAK [34,35,36] promote phosphorylation and activation of NKCC1. All four kinases, SPAK, OSR1, WNK1, and WNK4 are present in ORN cilia and are required for maximal activation of the transporter. The five proteins form a supramolecular complex: SPAK and OSR1 bind toNKCC1 [37], whereasWNK1 andWNK4 bind to SPAK/OSR1 [35,36]. It is interesting to note that the kinase WNK3 is not expressed in ORNs. WNK3 shows the highest expression in the brain, and it is thought to act as a Cl- sensor involved in protecting cells from excessive Cl- accumulation [37,38,39]. The absence of WNK3 from ORNs all points to the fact that these neurons are at dispense with means for intracellular Cl- reduction and thus instead optimize Cl- accumulation (Fig. 1).
Olfactory signaling is triggered when odors partition from the air into the olfactory mucus and bind to ORs in the membrane of olfactory cilia. Each OSN expresses one out of hundreds of distinct ORs that in vertebrates form the largest family of G protein-coupled receptors (GPCRs) and comprise approximately 250-1,200 functional OR genes [40]. Binding of an odorant to an OR is transduced into an electrical signal via the sequential activation of three major signaling proteins. ORs trigger activation of the olfactory G protein (Golf), an olfactory isoform of the stimulatory Gs protein [41], which in turn activates the MOE-specific [3] adenyl cyclase type III (ACIII) leading to the production of cAMP. The intracellular rise in cAMP levels then opens cyclic nucleotide-gated (CNG) cation channels that allow for influx of Na+ and Ca2+ from the mucus into the ciliary lumen thereby depolarizing the ciliary membrane. An additional crucial amplification step follows the initial depolarization by CNG channels: the influx of Ca2+ activates calcium activated chloride channels (CACC) that mediate a secondary depolarizing Cl- efflux, which accounts for the majority of the receptor current (Fig. 1). The transduction current carried by these olfactory ion channels induces slow and graded receptor potentials which summate at the soma and, upon reaching the threshold level, eventually trigger action potentials and neuronal activity.
Olfactory signal transduction has been intensively studied and its importance for olfaction has been clearly established with knockout studies. In OSNs from mice lacking Golf [42], ACIII [43,44] or the α-subunit of the CNG channel CNGA2 [4,45], receptor potentials in response to odorants were virtually abolished in electro olfactogram (EOG) measurements. These knockout mice showed major deficits in olfactory behavioral tasks and different odor-guided behaviors were impaired. This included the lack of suckling behavior, which manifests in high postnatal death rates due to the inability to feed, and deficits in sexual and aggressive behavior that have been reported for CNGA2 and ACIII knockout mice [4,44,45]. The lack of olfactory input in these mice was also reflected in reduced expression of activity-dependent markers in the olfactory bulb [46,47].
Olfactory signal termination and adaptation ensures high olfactory sensitivity during continuous or repetitive odor stimulation and allows for odor detection in the presence of background odors. Studies on the underlying molecular mechanisms at the level of signal transduction in the cilia have revealed a major role of Ca2+. Ca2+ entering through CNG channels acts on different elements of the odor transduction cascade. First, the CNG channels themselves are subject to feedback inhibition by CaM [48], a mechanism that primarily functions to terminate OSN responses [49] (Fig. 1). Second, Ca2+ leads to activation of CaM-dependent protein kinase II (CaMKII), which is thought to shape activation kinetics since its inhibition results in changes of the latter (Fig. 1). Third, Ca2+ extrusion mechanisms play a crucial role in signal termination and adaptation. The activity of sodium-dependent Ca2+ exchangers [50,51], ATP-dependent Ca2+ pumps [52,53], and the potassium-dependent Na+/Ca2+ exchan ger [54] have been implicated in the process (Fig. 1). The olfactory response is also shaped by cAMP removal by phosphodiesterase (PDE). PD E1C and PDE4A have been detected in OSNs and when both these enzymes are lacking, response termination is slowed [55] (Fig. 1).
A review of the literature on olfactory transduction, during the last decade, shows tremendous progress in this field. Previous intractable questions about signal transduction in ORN's have been answered which has provided new findings relevant for understanding transduction mechanisms in other types of neurons and sensory receptor cells. However, despite these remarkable findings, one cannot deny the fact that there is still much left to be discovered. The olfactory system is ripe with opportunities for the imaginative investigator. We still have little knowledge about how information from each olfactory subsystem is integrated within the higher center of the brain. Of particular interest is to determine how olfactory information can be integrated with taste, and soma-tosensory system, all of which can impact perception. Although humans have developed a complex olfactory world, they lack some subsystems present in rodents e.g., the Accessory Olfactory System. A complete understanding of the human olfactory subsystems is needed if we are to uncover the full extent of the human chemosensory world. Recent astounding development in stem cell research and technical advance in imaging could open our eyes in observing the detailed structure of insertion of each component in the olfactory epithelium and immaculate expression pattern of the responsible ion channels. With these technological advances we can come a step closer to the clear picture of olfactory perception.
Figures and Tables
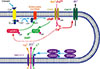 | Fig. 1Graphical representation of anion-based signal amplification in OSN cilia. OR, odorant receptor; CNG, cyclic nucleotide-gated channel; CaM, calmodulin; PDE, phosphodiesterase. CACC, Ca2+-activated Cl- channel; NKCC1, Na+/K+/2Cl- cotransporter; OSR1, oxidative stress-responsive kinase-1; SPAK, STE20/SPS1-related proline/alanine-rich kinase; WNK1 and WNK4, with no lysine (K) kinases. |
References
1. Mombaerts P. Genes and ligands for odorant, vomeronasal and taste receptors. Nat Rev Neurosci. 2004; 5:263–278.

2. Firestein S, Picco C, Menini A. The relation between stimulus and response in olfactory receptor cells of the tiger salamander. J Physiol. 1993; 468:1–10.

3. Bakalyar HA, Reed RR. Identification of a specialized adenylyl cyclase that may mediate odorant detection. Science. 1990; 250:1403–1406.

4. Brunet LJ, Gold GH, Ngai J. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron. 1996; 17:681–693.

5. Takeuchi H, Kurahashi T. Identification of second messenger mediating signal transduction in the olfactory receptor cell. J Gen Physiol. 2003; 122:557–567.

6. Zheng J, Zagotta WN. Stoichiometry and assembly of olfactory cyclic nucleotide-gated channels. Neuron. 2004; 42:411–421.

7. Dhallan RS, Yau KW, Schrader KA, Reed RR. Primary structure and functional expression of a cyclic nucleotide-activated channel from olfactory neurons. Nature. 1990; 347:184–187.

9. Kurahashi T, Yau KW. Co-existence of cationic and chloride components in odorant-induced current of vertebrate olfactory receptor cells. Nature. 1993; 363:71–74.

10. Lowe G, Gold GH. Nonlinear amplification by calcium-dependent chloride channels in olfactory receptor cells. Nature. 1993; 366:283–286.

11. Kashiwayanagi M, Miyake M, Kurihara K. Voltage-dependent Ca2+ channel and Na+ channel in frog taste cells. Am J Physiol. 1983; 244:C82–C88.

12. Kleene SJ, Pun RY. Persistence of the olfactory receptor current in a wide variety of extracellular environments. J Neurophysiol. 1996; 75:1386–1391.

13. Zufall F, Leinders-Zufall T. The cellular and molecular basis of odor adaptation. Chem Senses. 2000; 25:473–481.

14. Leinders-Zufall T, Greer CA, Shepherd GM, Zufall F. Imaging odor-induced calcium transients in single olfactory cilia: specificity of activation and role in transduction. J Neurosci. 1998; 18:5630–5639.

15. Leinders-Zufall T, Rand MN, Shepherd GM, Greer CA, Zufall F. Calcium entry through cyclic nucleotide-gated channels in individual cilia of olfactory receptor cells: spatiotemporal dynamics. J Neurosci. 1997; 17:4136–4148.

16. Zhainazarov AB, Ache BW. Odor-induced currents in Xenopus olfactory receptor cells measured with perforated-patch recording. J Neurophysiol. 1995; 74:479–483.

17. Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008; 322:590–594.

18. Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008; 134:1019–1029.

19. Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008; 455:1210–1215.

20. Yu TT, McIntyre JC, Bose SC, Hardin D, Owen MC, McClintock TS. Differentially expressed transcripts from phenotypically identified olfactory sensory neurons. J Comp Neurol. 2005; 483:251–262.

21. Stephan AB, Shum EY, Hirsh S, Cygnar KD, Reisert J, Zhao H. ANO2 is the cilial calcium-activated chloride channel that may mediate olfactory amplification. Proc Natl Acad Sci U S A. 2009; 106:11776–11781.

22. Mayer U, Kuller A, Daiber PC, Neudorf I, Warnken U, Schnolzer M, et al. The proteome of rat olfactory sensory cilia. Proteomics. 2009; 9:322–334.

23. Sagheddu C, Boccaccio A, Dibattista M, Montani G, Tirindelli R, Menini A. Calcium concentration jumps reveal dynamic ion selectivity of calcium-activated chloride currents in mouse olfactory sensory neurons and TMEM16b-transfected HEK 293T cells. J Physiol. 2010; 588:4189–4204.

24. Yan C, Zhao AZ, Bentley JK, Loughney K, Ferguson K, Beavo JA. Molecular cloning and characterization of a calmodulin-dependent phosphodiesterase enriched in olfactory sensory neurons. Proc Natl Acad Sci U S A. 1995; 92:9677–9681.

25. Wei F, Qiu CS, Liauw J, Robinson DA, Ho N, Chatila T, et al. Calcium calmodulin-dependent protein kinase IV is required for fear memory. Nat Neurosci. 2002; 5:573–579.

26. Bradley J, Bonigk W, Yau KW, Frings S. Calmodulin permanently associates with rat olfactory CNG channels under native conditions. Nat Neurosci. 2004; 7:705–710.

27. Leblanc N, Ledoux J, Saleh S, Sanguinetti A, Angermann J, O'Driscoll K, et al. Regulation of calcium-activated chloride channels in smooth muscle cells: a complex picture is emerging. Can J Physiol Pharmacol. 2005; 83:541–556.

28. Reisert J, Lai J, Yau KW, Bradley J. Mechanism of the excitatory Cl- response in mouse olfactory receptor neurons. Neuron. 2005; 45:553–561.

29. Kaneko H, Putzier I, Frings S, Kaupp UB, Gensch T. Chloride accumulation in mammalian olfactory sensory neurons. J Neurosci. 2004; 24:7931–7938.

30. Nickell WT, Kleene NK, Kleene SJ. Mechanisms of neuronal chloride accumulation in intact mouse olfactory epithelium. J Physiol. 2007; 583:1005–1020.

31. Nickell WT, Kleene NK, Gesteland RC, Kleene SJ. Neuronal chloride accumulation in olfactory epithelium of mice lacking NKCC1. J Neurophysiol. 2006; 95:2003–2006.

32. Delpire E, Gagnon KB. SPAK and OSR1: STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem J. 2008; 409:321–331.

33. Kahle KT, Ring AM, Lifton RP. Molecular physiology of the WNK kinases. Annu Rev Physiol. 2008; 70:329–355.

34. Gagnon KB, England R, Delpire E. A single binding motif is required for SPAK activation of the Na-K-2Cl cotransporter. Cell Physiol Biochem. 2007; 20:131–142.

35. Anselmo AN, Earnest S, Chen W, Juang YC, Kim SC, Zhao Y, et al. WNK1 and OSR1 regulate the Na+, K+, 2Cl- cotransporter in HeLa cells. Proc Natl Acad Sci U S A. 2006; 103:10883–10888.

36. Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J. 2005; 391:17–24.

37. Ponce-Coria J, San-Cristobal P, Kahle KT, Vazquez N, Pacheco-Alvarez D, de Los, et al. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci U S A. 2008; 105:8458–8463.

38. de Los Heros P, Kahle KT, Rinehart J, Bobadilla NA, Vazquez N, San Cristobal P, et al. WNK3 bypasses the tonicity requirement for K-Cl cotransporter activation via a phosphatase-dependent pathway. Proc Natl Acad Sci U S A. 2006; 103:1976–1981.

39. Kahle KT, Rinehart J, de Los Heros P, Louvi A, Meade P, Vazquez N, et al. WNK3 modulates transport of Cl- in and out of cells: implications for control of cell volume and neuronal excitability. Proc Natl Acad Sci U S A. 2005; 102:16783–16788.

40. Niimura Y, Nei M. Extensive gains and losses of olfactory receptor genes in mammalian evolution. PLoS One. 2007; 2:e708.

41. Fung YK, Reed JA, Lau YS. Prenatal cocaine exposure fails to modify neurobehavioral responses and the striatal dopaminergic system in newborn rats. Gen Pharmacol. 1989; 20:689–693.

42. Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in G(olf) are anosmic. Neuron. 1998; 20:69–81.

43. Wong ST, Trinh K, Hacker B, Chan GC, Lowe G, Gaggar A, et al. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron. 2000; 27:487–497.

44. Wang Z, Balet Sindreu C, Li V, Nudelman A, Chan GC, Storm DR. Pheromone detection in male mice depends on signaling through the type 3 adenylyl cyclase in the main olfactory epithelium. J Neurosci. 2006; 26:7375–7379.

45. Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci. 2005; 8:1660–1662.

46. Trinh K, Storm DR. Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nat Neurosci. 2003; 6:519–525.

47. Baker H, Cummings DM, Munger SD, Margolis JW, Franzen L, Reed RR, et al. Targeted deletion of a cyclic nucleotide-gated channel subunit (OCNC1): biochemical and morphological consequences in adult mice. J Neurosci. 1999; 19:9313–9321.

48. Bradley J, Reisert J, Frings S. Regulation of cyclic nucleotide-gated channels. Curr Opin Neurobiol. 2005; 15:343–349.

49. Song Y, Cygnar KD, Sagdullaev B, Valley M, Hirsh S, Stephan A, et al. Olfactory CNG channel desensitization by Ca2+/CaM via the B1b subunit affects response termination but not sensitivity to recurring stimulation. Neuron. 2008; 58:374–386.

50. Pyrski M, Koo JH, Polumuri SK, Ruknudin AM, Margolis JW, Schulze DH, et al. Sodium/calcium exchanger expression in the mouse and rat olfactory systems. J Comp Neurol. 2007; 501:944–958.

51. Reisert J, Matthews HR. Na+-dependent Ca2+ extrusion governs response recovery in frog olfactory receptor cells. J Gen Physiol. 1998; 112:529–535.

52. Antolin S, Reisert J, Matthews HR. Olfactory response termination involves Ca2+-ATPase in vertebrate olfactory receptor neuron cilia. J Gen Physiol. 2010; 135:367–378.

53. Kleene SJ. Limits of calcium clearance by plasma membrane calcium ATPase in olfactory cilia. PLoS One. 2009; 4:e5266.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download