Abstract
Smell used to be a common diagnostic tool in medicine, and physicians were trained to use their sense of smell during their medical training. Latterly, odor disgnostics have been relegated to secondary status as a diagnostic method. Array-based gas sensors ("Electronic Nose") now offer the potential of a robust analytical approach to exhaled breath analysis for medical use. Many diseases are accompanied by characteristic odor, and their recognition can provide diagonostic clues, guide the laboratory evaluation, and affect the choice of immediate therapy. We are developing an intelligent sensor system for non-invasive health care monitoring combined laboratory based sensor module, pattern recognition subsystem and non-invasive sampling of volatile emitted from a patient into a highly intelligent sensor system that allows the rapid processing of these samples. It is capable to assist early and rapid disgnosis of changes in state of patient, and aid decision making by medical personnel in the treatment of such patients. In this paper, we introduce exhaled breath analysis for potential primary lung disease screening using electronic nose system incorporating an automated solid-phase microextraction (SPME) desorption to enable the system to be used. Aiming to increase the sensitivity, SPME preconcentration is used for sampling of headspace air and the response of sensor module to variable concentration of volatile emitted from SPME fiber is evaluated. The initial result shows the distinguished difference between the lung cancer patients and healthy normal individuals according to the analysis of the respective expiratory gases.
There are many respiratory diseases including lung cancer, chronic obstructive pulmonary disease (COPD), asthma, and so on. Among them, lung cancer and COPD are leading causes of death in the world. However, diagnosis of those diseases vary often late in the course of the diseases since available diagonostics methods are not sufficiently sensititive and specific. There is evidence to suggest that particular diseases can be detected by odor analysis of exhaled breath, because smell has been used to be a common diagnostic tool in medicine and physicians were trained to use their sense of smell from Hippocrates era. It was observed that patients with uncontrolled diabetes had the smell of rotten apples because of the acetone in their breath [1].
Human breath contains more than thousands of different compounds including large number of Volatile Organic Compounds (VOCs). Exhaled breath is produced by different biochemical processes including those that are released during disease cell metabolism. There are several lines of evidence showing that metabolic pathways in diseased cells produce different volatile compounds than non-diseased tissue. This imples that breath analysis can be used as a diagnostic tool because increased or decreased concentrations of some compounds are associated with various diseases or an altered metabolism.
Breath compounds are present in parts-per-billion (ppb) with respect to volume or even lower quantities. Different analytical techniques have been used for analysis of exhaled breath. One of the most useful techniques is gas chromatography and mass spectrometer (GC-MS). This technique gives the most detailed analytical information. Analytical results from GC-MS have shown more than two hundreds of VOCs detected from human exhaled breath, especially alkanes and aldehydes have shown relatively increased levels in the breath of lung cancer patients [2]. Although GC-MS gives a wealth of information for research, the method has several prominent disadvantages for use as a clinical point-of-care application. First and foremost, it requires sophisticated, expensive, and bulky equipment that would only be available in large, well-equipped laboratories. Second, the interpretation of the GC-MS results is challenging and requires much expertise. All in all, the method would be too time-consuming and expensive for lung disease screening [3]. Unlike traditional breath analysis techniques, the electronic nose system, which is an instrument comprises an array of chemical sensors with partial specificity and an appropriate pattern recognition system capable of recognizing simple or complex odors [4], is gaining popularity in discriminating lung diseases for screening following exhaled breath analysis.
Mimicking the human olfactory system is required to develop better electronic noses in the area of sensor development, pre-processing technqiues and pattern recognition. Electronic noses contain an odor delivery system, array of chemically sensitive sensors with partial specificity to a range of odorants, signal preparation for sensor and array processors, a pattern recognition engine and some form of an odor representation scheme. The major process between the mammalian nose and an electronic nose is shown in the Table 1, and can be illustrated as follows [5]:
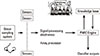
The odor molecules are drawn into the e-nose using sampling techniques such as headspace sampling, diffusion methods, bubblers or pre-concentrators [6]. The odor sample is drawn across the sensor array and induces a reversible physical and/or chemical change in the sensing material, which causes an associated change in electrical properties, such as conductivity. Each sensor (cell) in array can behave like a receptor by responding to different odors to varying degrees and transferred into electrical signals, which are preprocessed before identification by a pattern recognition system. The electronic nose system is designed so that the overall response pattern from the array, is unique for a given odor, in a family of odors to be considered by the system. The Fig. 1 illustrates the basic elements that are working in an artificial olfaction system.
We are developing a fast reliable method for screening of lung disases using electronic nose technology. The system for screening lung disases such as lung cancer was made using metal oxide type chemical sensor array with solid phase microextraction (SPME) fiber to detect low concentration for exhaled breath [7]. We collected real samples from patients to determine whether samples can be differentiated on the basis of the volatile compound present.
The strategy is to use SPME as a method of sampling and preconcentration of volatiles from headspace. SPME is a technique to sample and preconcentrate the headspace using a fiber with a hydrophilic and/or hydrophobic coating [8]. The fiber adsorbs and/or absorbs the analytes in the headspace, and it is easy to handle. The system was tested by measuring the exhaled breath for lung cancer patients as well as healthy subjects throughout the experiment. Among the lung diseases, lung cancer accounts for 28% of cancer-related deaths and 1.3 million people worldwide deaths every year [9]. More additionally, it is very difficult to screen at the very early stage for lung cancer.
The key marker volatiles were detected and the volatile compounds emitted from exhaled breath. The SPME technique combined with GC-MS provides the possibility of early recognition of lung cancer.
The results obtained from this GC-MS study allowed us to build a portable system for exhaled breath analysis for lung cancer using an array of gas sensors, to be used for point care monitoring of patients.
Sensors based on metal oxides were used, modified, and refined aimimg to detect the most probable key markers for lung cancer from exhaled breath found in the literature [9]. For samplings from exhaled breath from patients and controls, a SPME approach was used for preconcentration of the low concentrations of volatile compounds emitted. An instrument was constructed that incorporated an automated SPME desorption system, sensor array electronic, and data processing to enable the system to be used for clinical validation. Samples of patients and controls were analyzed in a preliminary study using an instrument based on electronic nose technology. Primary results from metal oxide sensor array give indications that lung cancer patients may be discriminated from healthy individuals.
We collected the exhaled breath of the lung cancer patients and healthy subjects using a tedlar bag, using sizes that were 1L-3L. The sampling collections for exhaled breath have been performed in the morning before any food in-take and after gargling with purified water. Individuals were required to breathe deeply into the bag. A mouthpiece and aerobic filter for filtering impurities were connected to the tedlar bag while the breath samples were collected. Also, the bag had airtight valves to in order to prevent any diffusion of external air from the bag. The sample was transferred to an analytical instrument unit for chemical analysis of breath samples. Fig. 2 shows the sampling bag used in collection for exhaled breath.
Qualitative analysis of extracted volatiles from exhaled breath samples was performed with GC-MS (GC: HP5890, MSD:HP5972) apparatus in combination with SPME. The inlet was operated in split less mode at 280℃, and the SPME fibers remained in the inlet for 5 minutes. The SPME fiber 65 µm polydimethylsiloxane/divinylbenzene (PDMS/DVB) was exposed to the breath sample for 30 minutes. A search of libraries allowed us to identify the volatiles produced by samples from exhaled breath that were then extracted.
An electronic nose prototype was developed comparising an array of chemcial sensors, chamber, data acquisition system with a microprocessor, and sampling system for SPME fiber operation. Sensors based on metal oxide were produced, modified, and refined with an aim to detect the most probable key markers for lung cancer most frequentely found in GC-MS results. The criteria for selection of sensors was determined by sensitivity and selectivity of sensors to a limited number of the volatile compounds produced by exhaled breath from lung cancer patients.
The sensors in the system are commercially available metal oxide sensors fabricated by Figaro (TGS 2600, TGS 2620, TGS 2602, and TGS 2610), Japan [10].
The instrumentation incorporated an SPME sampler, which allowed the SPME fibers to be contained and used for sampling via a plunger and locking system. The device acts as a mechanism for preconcentration of the sample, which effectively increase sensitivity of electronic nose measurement.
The collection of exhaled breath of patients and controls was carried out using a 1.5 L tedlar bag in connection with mouthpiece having a saliva trap to prevent humidity. The SPME fiber coated 65 µm polydimethylsiloxane/divinylbenzene (PDMS/DVB) was inserted into the tedlar bag and exposed to the collected breath at an ambient temperature. The components of exhaled breath were allowed to adhere to the SPME fiber. After sampling, the fiber was placed into a loader in the Electronic Nose/SPME system that was controlled using an embedded processor and specially designed control software. All systems were controlled by touch screen panels for easy use. The system exposed the fiber to the heated sensor array desorbing the volatiles off the fiber directly onto the sensors in the chamber. The sensor system was linked to data processing software developed for real-time data acquisition of incoming data. The response of each individual sensor was measured over time after exposure to the volatiles. The electronic nose prototype with combined sensor array chamber and SPME fiber tray for real-time lung disease screening is shown in Fig. 3.
Table 2 shows the GC-MS results for breath samples from patients and healthy subjects based on SPME technique. The smokers in the group showed benzene and toluene in their breath samples, while those were not found in non-smokers'. The important volatile substances which characterized the lung cancer patients were decane, tridecane, octdecane, heneicosane, and undecane etc. These volatile components are similar to those found in previous contributions [11].
The results obtained from GC-MS show that the collection of breath samples enable screening of lung diseases patients. Fig. 4 illustrates the principal component analysis (PCA) obtained from the electronic nose using the SPME technique from breath samples taken from small samples of lung cancer patients and healthy subjects. This analysis clearly indicates that breath analysis could be an important element in screening for lung cancer. So, it is important to properly analyze the breath test results to distinguish between healthy people and lung cancer sufferers.
The PCA results of data obtained from the electronic nose/SPME system give the indication that lung cancer patients may be discriminated from healthy peoples.
Based on an array of electronic gas sensors, an odor recognition system was developed for potential screening of lung disease at the early stage of cancer. It was proved by GC-MS studies that exhaled breath samples can be distinguished from each other by means of a limited set of key volatile products.
To increase reliability of exhaled breath identification, SPME preconcentration was used for sampling the headspace air. Respon-ses to variable concentrations of volatiles emitted from the SPME fiber were processed for evaluation of output parameters of the sensor module.
Discrimination between lung cancer patients and healthy controls was obtained by PCA analysis of dynamic parameters of sensor responses to the headspace air of samples collected by exhaled breath.
Primary results at this stage appear to be promising. However, more clinical validation with instruments is needed in hospitals where patients with lung diseases are treated.
Figures and Tables
 | Fig. 3A chemcial sensor array chamber and electronic nose prototype system with SPME fiber tray for real-time lung disease screening. |
 | Fig. 4Principal component analysis for exhaled breath samples from healthy and lung cancer individuals; control: healty people, LC-AD: adenocarcinoma, LC-NSCLC: non small cell lung cancer, LC-SQCC: squamous cell carcinoma (In the case of NSCLC and SQCC, the data looks different but it located same cluster in LC patients). |
ACKNOWLEDGMENT
This research was supported by the Converging Research Center Program through the Ministry of Science, ICT and Future Planning, Korea (No. 2013K000364).
References
2. Hakim M, Broza YY, Barash O, Peled N, Phillips M, Amann A, et al. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem Rev. 2012; 112:5949–5966.

3. Tisch U, Billan S, Ilouze M, Phillips M, Peled N, Haick H. Volatile organic compounds in exhaled breath as biomarkers for the early detection and screening of lung cancer. CML-Lung Cancer. 2012; 5:107–117.

4. Gradner J, Bartlett P. Electronic noses principles and applications. Meas Sci Technol. 2000; 11:20–25.
5. Bishop L. Application of chemometric methods applied to breath analysis. Bedfordshire: Cranfield University;2006. p. 8–11.
6. Pearce TC. Computational parallels between the biological olfactory pathway and its analogue 'the electronic nose': Part II. Sensor-based machine olfaction. Biosystems. 1997; 41:69–90.

7. Cesare F, Pantalei S, Zampetti E, Macagnano A. Electronic nose and SPME techniques to monitor phenanthrene biodegradation in soil. Sensor Actuat B-Chem. 2008; 131:63–70.

8. Krizek TJ, Robson MC. Evolution of quantitative bacteriology in wound management. Am J Surg. 1975; 130:579–584.

9. Peng G, Tisch U, Adams O, Hakim M, Shehada N, Broza YY, et al. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nat Nanotechnol. 2009; 4:669–673.

10. Figaro USA Inc. The Leader in Semiconductor Gas Sensor Technology. West Lake Ave(USA): Figaro USA Inc;c2013. cited 2014 May 3. Available from: http://www.figarosensor.com/.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download