INTRODUCTION
Sense of smell, often called olfaction, is a significant way for vertebrate animals that have 'the nose' to recognize the chemical signals from their environment. Generally, olfaction of the nose is the most important sense for the majority of vertebrate animals to maintain their survival with the exception of several mammals, including humans. Human's ability to smell is significantly weaker than that of most of other animals, and people with anosmia have much less of a life handicap than those who are blind or deaf. Probably for this reason, few clinical efforts to treat the olfactory disorders have been made until scientists published their notable research about olfaction, one of which won the 2004 Nobel Prize [1]. Here, basic knowledge about human olfaction will be introduced briefly, followed by information about some landmark studies and current debates in the field.
GROSS AND CELLULAR ANATOMY OF THE HUMAN NOSE AS AN ORGAN FOR SMELLING
In fish, the nose is devoted to olfaction only. Other vertebrates breathe and smell with their noses. In a human nose, the nostrils and nasal airways are divided into two by median structures, the columella and the nasal septum. The lateral wall of each nasal cavity is delineated by the curves of the inferior, middle, and superior turbinates. The majority of mucosa lining the structures of the nasal cavity is respiratory epithelium, which functions in mucociliary clearance, swelling, and humidification, supporting the nose as a respiratory organ. The mucosa lining for olfaction, called human olfactory epithelium, about 2-10 cm2 in size, is located at the olfactory cleft just beneath the cribriform plate and partially at the superior aspect of the superior turbinate [2,3].
Microscopically, the olfactory epithelium is a layered structure of neuronal and nonneuronal cells. Olfactory receptor neurons (ORNs) are the most important cells functionally because they are the primary sensory cells of olfaction, like the retinal cells of the eye or the hair cells of the cochlea. ORNs are bipolar cells with bidirectional neuronal projections. The apical projection from an ORN is a dendrite projecting to the luminal surface terminating in a swelling called an olfactory knob. Projecting from the knob are several microvilli called olfactory cilia, which are 5-20 µm long and nonmotile, extending into a thick layer of mucus and providing an extensive surface area for contact with odorant molecules. The basal surface of an ORN gives rise to a small-diameter unmyelinated axon. The convergent bundles of axons from the ORNs pass through the cribriform plate, and then reach the olfactory bulb just beyond the plate to transmit olfactory sense input centrally. Basal cells are located at the basal lamina of the epithelium as a thin layer. They can give rise to progenitor cells that retain the ability to divide throughout life. The human olfactory epithelium is replaced every 3-5 weeks due to the constant division of progenitor cells, so there are mature and immature ORNs of different life stages together in an olfactory epithelium. The newly replacing mature ORN expresses the same cellular properties in terms of odor sensitivity, preference, and axonal projection as the former one [4]. Some scientists insist that progenitor cells are a kind of basal cell that has the neural stem cell activity. They call the progenitor cells located more superficially 'globose' basal cells, and the conventional basal cells are termed 'horizontal' basal cells [5,6]. Mature ORN expresses olfactory marker protein (OMP), which plays a role in functional maturation of ORN and signal transduction in ORN [7]. Sustentacular cells are a kind of nonneuronal cell shown as pseudostratified columnar cells. Their physiologic role is regarded as metabolic and physical support for the neural cells. Another kind of nonneuronal cell in the olfactory epithelium is the microvillar cells, also called brush cells, which have nonmotile microvilli on their apical surface. The basal surface of these cells are in contact with the terminal branches of the trigeminal nerve, so their role might be general sensation or trigeminal olfaction to detect the noxious stimuli, but this has not been proven [8]. The mucus covering the olfactory epithelium is 5-30 µm thick and is produced by Bowman's glands, secretory glands located at the submucosa. The mucus contains electrolytes and a variety of mucopolysaccharides and proteins, including odorant-binding proteins. The role of the odorant-binding protein is to enhance the solubility of hydrophobic odorants. The mucus traps and dissolves odorants for the olfactory cilia of ORNs, and controls the ionic milieu of the olfactory epithelium [9].
OLFACTORY RECEPTORS
Membranes of the olfactory cilia present olfactory receptors (ORs). The ORs are members of the G-protein-coupled receptor (GPCR) family, which are coupled to GTP-binding regulatory proteins (G-proteins). The OR proteins are transmembrane receptor proteins, which have seven transmembrane domains with about 20-25 amino acids in each domain and are about 350 amino acids in total length. The amino acid sequences of various ORs are highly conserved in G-protein binding region, while hypervariable in ligand binding regions. OR proteins represent the largest gene family in vertebrates. Genomic DNA analyses have provided estimates for the size of the putative OR gene family repertoire of about 1,000 in mammalian animals, about 2-3% in the genome. However, studies of the completed genome data from humans and mice suggest that there are about one-third as many potentially functional OR sequences in the human genome compared to the mouse genome because two-thirds of OR gene sequences are pseudogenes in the human genome [10]. Furthermore, the vast majority of ORs still remain 'orphan' receptors, in that their ligands are not yet known [11]. Two major classes of olfactory receptors have been identified in humans; class I OR families for water-soluble odorants (fish-like) and class II OR families for air-borne hydrophobic odorants (mammalian-like) [12]. Currently available data suggest that each ORN expresses only a single type of functional OR, and that only one allele of a given OR is expressed [13,14]. The molecular mechanisms that regulate how each mature ORN selects a particular OR and how their expression pattern is maintained in the face of ongoing ORN replacement remain to be explained. In 2000, the vomeronasal type 1 receptor, V1R, which is a GPCR and the receptor for pheromones in most of animals, was found in the human olfactory mucosa [15]. However, the vomeronasal organ and the nervus terminalis that connects the vomeronasal organ and the limbic system are just vestigial organs in humans, and the role of these receptors in humans is disputed. Since 2006, another class of odorant receptors has been found, called the trace amine-associated receptors (TAAR) which exist for volatile amines frequently present in the urine of animals [16]. However, the role of TAAR in the human olfactory mucosa is still unknown. An OR displays affinity not for only a kind of specific molecule but for a range of odor molecules, and conversely, a single odorant molecule may bind to a number of different olfactory receptors with varying affinities [17]. There is an important mismatch of numbers between hundreds of expressed receptors and thousands of different odors. Combinatorial model theory is the most supported and proven hypothesis to explain such a mismatch gap in odor discrimination.
SIGNAL TRANSDUCTION IN OLFACTORY RECEPTOR NEURON
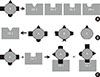
With binding of an odorant, the OR at the ciliary membrane changes its structure to activate the G-protein of olfactory type (Golf) bound inside the ORN. The activated Golf in turn activates adenylate cyclase, which converts adenosine triphosphate (ATP) into cyclic adenosine monophosphate (cAMP), the key secondary messenger of the transduction cascade in ORN (Fig. 1). The cAMP opens cyclic nucleotide-gated (CNG) ion channels which allow an influx of calcium and sodium ions into the ORN, resulting in depolarization, leading to an action potential, which carries the information through the neuron centrally [18]. Knockout mice lacking functional CNG channels are unable to detect most [19], but not all odors [20]. These results show that the cAMP-linked pathway is a major, but not the only mechanism for odor detection by which ORNs respond to odorant stimuli. The inositol triphosphate (IP3)-linked pathway is another known mechanism of ORN stimulation in mammals including humans [21,22]. This pathway uses IP3 as a secondary messenger instead of cAMP. There is another type of G-protein which can activate the membrane-bound enzyme phospholipase C. Together with diacyl glycerol, IP3 is converted from a lipid phosphatidylinositol 4,5-bisphosphate in the plasma membrane by phospholipase C. A study comparing density and function between CNG and IP3-gated channels in rodent ORNs implies that the cAMP-linked pathway dominates the IP3-linked pathway in mammalian olfactory transduction [23]. Some studies support the existence of multiple mechanisms besides the cAMP and the IP3-linked pathways [24]. The influx of calcium ions into the ORN through CNG channels or IP3-gated channels triggers an action potential with the amplification of voltage-gated calcium channels, which leads to the further influx of calcium ions and the opening of calcium-dependent chloride channels to enhance the efflux of chloride ions [25,26,27]. Calcium and cAMP activate not only ion channels but also various protein kinases, including protein kinase A and calcium/calmodulin-dependent kinase II that are involved in the termination of the odorant signal [28,29]. After depolarization, protein kinase acts as a phosphorylase to inactivate the ion channels and other cascade components, resulting in the adaptation of the ORN to odorant stimulus. Intracellular calcium ions are removed through sodium/calcium exchangers located at the ciliary layer of the ORN [30]. 2,4,6-Trichloroanisole can be the suppressor of olfactory signal transduction by suppressing the CNG channels, causing the reduction of flavor [31].
DETECTION AND DISCRIMINATION OF ODOR: TWO DIFFERENT THEORIES
1. Shape theory
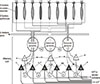
This theory, also called the steric theory, states that a particular odor of a molecule is due to a structural specificity between the odorant and the OR, like a key-and-lock. The basic idea of this theory has been developed since 1949. In 1991, Richard Axel and Linda Buck cloned and identified 1,000 GPCRs used for olfaction in mice [1]. Since all types of GPCRs currently known are activated through the binding of molecules with highly specific conformations or shapes, it is assumed that ORs operate in a similar fashion. This research caused the shape theory to dominate the field. The authors Buck and Axel won the Nobel Prize in 2004 with their related landmark research. Many other researchers have conducted numerous further studies about olfaction, and their results have solidified the shape theory. For example, one study provided direct proof that the cloned GPCRs of ORN were functionally real odor receptors by a morphologic gene expression study using green fluorescent protein and by the functional measurement of neuronal electrical activity in 1998 [32]. After Buck left Axel's laboratory, her further work suggested the presence of combinatorial receptor codes in odor perception, based on the fact that a single OR recognizes multiple odorants, and a single odorant is recognized by multiple ORs [33]. It means that different odorants are recognized by distinct combinations of receptors, and that the binding specificity between the epitope of odorant and the OR is present but not so high (Fig. 2). This combinatorial model was suggested and proved in other studies [34,35], and is believed as a main hypothesis in the perception and discrimination of odors though it does not yet explain everything. A recent theory, called weak shape theory or odotope theory, holds that receptors recognize only small structural features on each molecule, and the brain is responsible for processing the combined signal into an interpreted smell.
2. Vibration theory
This theory, originally from 1938, was proposed by Turin in 1996 and is also known as 'swipe card model' [36]. This theory proposed that when an OR binds an odorant, electron tunneling can occur across the binding site if the vibrational mode equals the energy gap between filled and empty electron levels. The electron tunneling activates the G-protein cascade. Receptors are therefore tuned to the vibrational frequency of particular odorants, like cones in the retina are tuned to particular wavelengths of light. According to this theory, the normal and deuterated versions of a compound should smell different because a bond with hydrogen and a bond with deuterium have different vibration energies due to their molecular weight differences [37]. Though it has been prov-ed in humans and animals [38,39,40], there have been strong disputes about this theory. Specifically, around the Nobel Prize winning year of 2004, this theory was considered as a theory of little credence, as mainstream scientists widely refuted it [41]. However, supporting evidence for this vibration theory has grown steadily. Recently, Turin published a study supporting his theory again [42]. In this paper, he accepted the results of refutation study that human subjects were unable to distinguish normal acetophenone from its deuterated form. However, the same subjects in his research could distinguish cyclopentadecanone from its fully deuterated analog. He suggested that the different test results between the two odor compounds must be caused by the difference in the number of hydrogen molecules, 28 in cyclopentadecanone compared to 8 in acetophenone. In the olfaction field, the shape theory is now more supported by mainstream scientists than the vibration theory, although the two theories are probably like the quantum theory and wave theory, which are both needed to describe the characteristics of light and electromagnetic waves in physics.
NEUROANATOMY AND CENTRAL NERVOUS PROCESSING OF OLFACTION
The olfactory bulb is located inside of the cranium on the cribriform plate bilaterally. The bulb and olfactory tract form the gross anatomical structure of intracranial CN I, the olfactory nerve. In the bulb, the terminal of axons from ORNs cluster in spherical structures called glomeruli, forming primary synapses connecting the higher neurons. Glomeruli are located most superficially at the bulb, forming the glomerular layer. There are about 1,200 glomeruli in a human olfactory bulb [43]. The neurotransmitter at the synapses of the glomeruli is glutamate, which is released from the axon terminal, where it activates the associated mitral cells. Between the glomeruli there are periglomerular cells which are short inhibitory interneurons among the glomerular synapses and the apical dendrites of mitral or tufted cells. The next level of synaptic processing in the olfactory bulb occurs in the external plexiform layer, between the glomerular layer and the mitral cell layer. The external plexiform layer contains astrocytes called tufted cells and interneurons. It does not contain many cell bodies, rather mostly dendrites of mitral cells and granule cells [44]. The mitral cell layer contains the cell bodies of mitral cells which are the principal neurons in the bulb. There are around 50,000 of mitral cells in a human olfactory bulb [43]. The apical dendrites of the mitral cells are connected to the glomeruli, and their axons merge together to form the lateral olfactory tract (LOT) present in the olfactory tract. They also have collateral connections for positive or negative neural feedback. Tufted cells, another type of principal neurons in the external plexiform layer, also have apical connections with glomeruli and central projection neurons like the mitral cells. However, their axons are relatively shorter than those of the mitral cells, and their projection targets are much more limited (Fig. 3). Inside the mitral cell layer, there are the internal plexiform layer and the granule cell layer. These layers contain granule cells that are GABAergic inhibitory interneurons to the cell bodies of mitral or tufted cells. They receive both contralateral and ipsilateral input. The LOT provides the only bulbar afferents to the human brain [45]. The brain target of LOT is called the primary olfactory cortex, which includes the pyriform cortex and other regions near the pyriform cortex such as the prepyriform, periamygdaloid, and entorhinal cortices. From the primary olfactory cortex, the axon of the higher neuron goes to the thalamus, then is further relayed to the orbitofrontal cortex for recognition, hypothalamus for neuroendocrine function, or other limbic systems including insula, basal ganglia, and hippocampus which have roles in the aspect of memory or emotion [46]. As we know from the neural anatomical connection (so-called lateralization of olfactory processes), olfaction is unique in that its direct connection to limbic system and because there is no thalamic intermediary before the primary cortex. Together, these features can explain why odors tend to have such strong emotional or memory associations. Moreover, there is a dissociation of olfactory processes, with involvement of the right hemisphere in memory processes and the left hemisphere in emotional processes [47]. From ORs to the glomeruli in olfactory bulbs, the distribution pattern of the neurons is a convergence. However, the pattern becomes divergent from the glomeruli to the higher neurons. So, it is yet to be discovered how the higher cortex responds to single or multiple odorant stimuli, or whether the combinatorial model is still valid at the higher neuronal levels in this convergent-divergent system. There have been three papers published from the Novel Prize winner Buck's laboratory that give some clues as to these questions since 2001 until 2006. Unfortunately, all three were retracted in 2008 and 2010 voluntarily by Buck and her co-authors, due to poor reproducibility of the experiments. However, other scientists are conducting research to understand the olfactory maps in higher neurons of mammalian animals or humans [48,49,50,51].
CONCLUSION
The past decades, especially since the early 1990s, have brought forth a greater understanding of the science behind human olfaction. Some of the research results have been supported from the mainstream and majority, and some have been controversial. Moreover, many more questions linger, leaving much to be discovered and unraveled in the field of olfaction science. Scientists and physicians should concentrate their efforts to obtain knowledge from basic olfaction science research, which can be the foundation for the treatment of incurable human sensorineural olfactory disorders.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download