Abstract
Lung cancer is characterized by accumulation of oncogene activation, inactivation of tumor suppressor genes and alteration of epigenetic changes. Fortunately, the past decade has seen remarkable development in molecular pathogenesis and management of lung cancer, especially adenocarcinoma. The discovery of the biologic and therapeutic importance of acquired genetic alterations in 2 genes that encode pharmacologically targetable tyrosine kinases involved in growth factor receptor signaling, epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK), has raised hope that targeted therapy will improve survival and quality of life of patients with lung cancer. Therefore, molecular testing to detect these 2 mutated genes is more important than ever and has changed the management of the patients with lung cancer and the role of pathologists. Furthermore, as most lung cancer patients present with advanced-stage disease at the time of diagnosis, it is important to detect targetable mutations using small tissue samples or cytology specimens. Here, the author summarizes the practical impact of the molecular testing of lung cancer and introduces the current knowledge of lung cancer pathology.
Figures and Tables
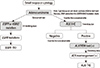 | Fig. 1Molecular testing guideline in pulmonary adenocarcinoma using small biopsy or cytology. Ref. 16 with permission from Elsevier. |
ACKNOWLEDGMENT
This work was supported by grants from the Korea Healthcare technology R&D project, Ministry of Health & Welfare, Republic of Korea (A111405) and the Basic Science Research Program through.
The National Research Foundation of Korea (NRF), which is funded by the government of Korea (2013-59757).
References
1. Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004; 304:1497–1500.

2. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004; 350:2129–2139.

3. Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011; 29:2866–2874.

4. Weinstein IB. Cancer. Addiction to oncogenes--the Achilles heal of cancer. Science. 2002; 297:63–64.
5. Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007; 448:561–566.

6. Palmer RH, Vernersson E, Grabbe C, Hallberg B. Anaplastic lymphoma kinase: signalling in development and disease. Biochem J. 2009; 420:345–361.

7. Wong DW, Leung EL, So KK, Tam IY, Sihoe AD, Cheng LC, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009; 115:1723–1733.

8. Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010; 363:1693–1703.
9. Camidge DR, Christensen J, Bang YJ, Shaw AT, Costa DB, Salgia R, et al. Addressing right drug/right target/right patient in phase I studies to accelerate bench to clinical benefit time: ALK gene rearrangements and the development of PF-02341066 in NSCLC. J Thorac Oncol. 2010; 5:S233.
10. Ladanyi M, Pao W. Lung adenocarcinoma: guiding EGFR-targeted therapy and beyond. Mod Pathol. 2008; 21:Suppl 2. S16–S22.

11. Paz-Ares LG, Altug S, Vaury AT, Jaime JC, Russo F, Visseren-Grul C. Treatment rationale and study design for a phase III, double-blind, placebo-controlled study of maintenance pemetrexed plus best supportive care versus best supportive care immediately following induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small cell lung cancer. BMC Cancer. 2010; 10:85.

12. Di Costanzo F, Mazzoni F, Micol Mela M, Antonuzzo L, Checcacci D, Saggese M, et al. Bevacizumab in non-small cell lung cancer. Drugs. 2008; 68:737–746.

13. Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC. Chapter 1, Tumours of the lung. Pathology and genetics: tumours of the lung, pleura, thymus, and heart. Lyon: IARC Press;2004. p. 9–124.
14. Loo PS, Thomas SC, Nicolson MC, Fyfe MN, Kerr KM. Subtyping of undifferentiated non-small cell carcinomas in bronchial biopsy specimens. J Thorac Oncol. 2010; 5:442–447.

15. Coate LE, John T, Tsao MS, Shepherd FA. Molecular predictive and prognostic markers in non-small-cell lung cancer. Lancet Oncol. 2009; 10:1001–1010.

16. Paik JH, Choi CM, Kim H, Jang SJ, Choe G, Kim DK, et al. Clinicopathologic implication of ALK rearrangement in surgically resected lung cancer: a proposal of diagnostic algorithm for ALK-rearranged adenocarcinoma. Lung Cancer. 2012; 76:403–409.

17. Sun PL, Seol H, Lee HJ, Yoo SB, Kim H, Xu X, et al. High incidence of EGFR mutations in Korean men smokers with no intratumoral heterogeneity of lung adenocarcinomas: correlation with histologic subtypes, EGFR/TTF-1 expressions, and clinical features. J Thorac Oncol. 2012; 7:323–330.

18. Yatabe Y, Matsuo K, Mitsudomi T. Heterogeneous distribution of EGFR mutations is extremely rare in lung adenocarcinoma. J Clin Oncol. 2011; 29:2972–2977.

19. Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013; 8:823–859.

20. Pao W, Ladanyi M. Epidermal growth factor receptor mutation testing in lung cancer: searching for the ideal method. Clin Cancer Res. 2007; 13:4954–4955.

21. Tanaka T, Nagai Y, Miyazawa H, Koyama N, Matsuoka S, Sutani A, et al. Reliability of the peptide nucleic acid-locked nucleic acid polymerase chain reaction clamp-based test for epidermal growth factor receptor mutations integrated into the clinical practice for non-small cell lung cancers. Cancer Sci. 2007; 98:246–252.

22. Kawada I, Soejima K, Watanabe H, Nakachi I, Yasuda H, Naoki K, et al. An alternative method for screening EGFR mutation using RFLP in non-small cell lung cancer patients. J Thorac Oncol. 2008; 3:1096–1103.

23. Kimura H, Fujiwara Y, Sone T, Kunitoh H, Tamura T, Kasahara K, et al. High sensitivity detection of epidermal growth factor receptor mutations in the pleural effusion of non-small cell lung cancer patients. Cancer Sci. 2006; 97:642–648.

24. Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009; 27:4247–4253.

25. Paik JH, Choe G, Kim H, Choe JY, Lee HJ, Lee CT, et al. Screening of anaplastic lymphoma kinase rearrangement by immunohistochemistry in non-small cell lung cancer: correlation with fluorescence in situ hybridization. J Thorac Oncol. 2011; 6:466–472.

26. Kim H, Jang SJ, Chung DH, Yoo SB, Sun P, Jin Y, et al. A comprehensive comparative analysis of the histomorphological features of ALK-rearranged lung adenocarcinoma based on driver oncogene mutations: frequent expression of epithelial-mesenchymal transition markers than other genotype. PLoS One. 2013; 8:e76999.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download