This article has been corrected. See "Corrigendum: Targeting Interleukin-17 and Th17 in Immune Inflammatory Diseases" in Volume 33 on page 137.
Abstract
Th17 cells (Th17) are a distinct lineage of CD4+ T cells that secrete high amounts of IL-17 under orphan nuclear receptor retinoic acid receptor-related orphan receptor γt (RORγt) which is a lineage-specific transcription factor. TGF-β and inflammatory cytokines, such as IL-6, IL-21, IL-1β, and IL-23, play central roles in the generation of Th17 cells. Th17 cells and their effector molecules, such as IL-17A, IL-17F, IL-21, IL-22, and CCL20, contribute to the progression and pathogenesis of several autoimmune and inflammatory diseases, such as rheumatoid arthritis, psoriasis, multiple sclerosis, inflammatory bowel disease and systemic lupus erythematosus. Studies of Th17 development and the effects of IL-17 signaling in autoimmune responses suggest a high potential for targeting this pathway in immune pathologies. In this review, we discuss Th17 biology in relation to autoinflammatory disorders and the various therapeutic strategies under investigation which target the IL-17-Th17 cell pathway in chronic inflammatory autoimmune disorders.
Figures and Tables
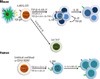 | Fig. 1Differentiation of Th17 cells in mouse and human. TGF-β and IL-6/21, or TGF-β3 and IL-6 required to induce the development of Th17 cells in mice. TNF and IL-1 are found to enhance TGF-mediated and IL-6-mediated differentiation of Th17 cells. Th17 cell differentiation was proposed to comprise three different stages (differentiation, amplification and stabilization). TGF-β, IL-21, IL-1, IL-6, and IL-23 are sufficient to induce the differentiation of human Th17 cells from naïve T cells. IL-1 and IL-6 are important for enhancing the expansion of differentiated and memory Th17 cells. IL, interleukin; Th17, IL-17-producing T helper; TGF-β, transforming growth factor beta; TNFα, tumour necrosis factor alpha; ROR, retinoic-acid-receptor-related orphan receptor; RORγt, ROR gamma t; RORα, ROR alpha; IRF4, interferon regulatory factor 4; STAT3, signal transducers and activators of transcription 3; APC, antigen presenting cell. |
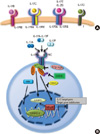 | Fig. 2IL-17 receptors and IL-17 signaling. (A) IL-17RA forms a complex with IL-17RB or IL-17RE to create the receptor for IL-17E (IL-25) and IL-17C, respectively.IL-17B and IL-17D bind to the monomeric receptors IL-17RB, while the receptor(s) for IL-17D has not yet been identified. (B) The IL 17R complex is composed of IL-17RA and IL-17RC. IL-17A or IL-17F homodimer or heterodimer can bind to IL-17R (the heterodimer of IL-17RA and IL-17RC). Binding of IL-17 to its receptor recruits adaptor protein ACT1. ACT1 is required for recruitment of TRAF6, which are essential upstream activators of NF-κB pathway. IL-17RA/ACT1/TRAF6 complex lead to the activation of NF-κB, and C/EBP. Also, ACT1-independent ERK activation contributes to IL-17R signaling. IL-17RA signaling can be negatively regulated by TRAF3, USP25 or SCFβ-TrCP. IL, interleukin; SCFβ-TrCP, SKP1-Cullin1-F-box beta-transducin repeat containing protein; TRAF, TNF-receptor-associated factor; ACT1, actin related gene 1; ERK, extracellular signal-regulated kinase; C/EBP, CCAAT/enhancer binding protein; NF-κB, nuclear factor kappa B; USP25, ubiquitin-specific protease 25. |
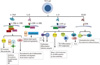 | Fig. 3Key functions of Th17 cytokines. Th17 cells produce several effector molecules, such as IL-17A, IL-17F, IL-21, IL-22, and CCL20. IL-17A and IL-17F play a role in the recruitment, activation, and migration of neutrophils and can target nonimmune cells, such as fibroblasts, endothelial cells, and epithelial cells, to induce pro-inflammatory mediators. IL-22 induces antimicrobial agents in keratinocytes and promotes epidermal hyperplasia. IL-21 stimulates proliferation/differentiation of CD8 T cells, and differentiate/antibody class switching of B cells. Also IL-21 induces differentiation and cytotoxic program of NK and NKT cells, and IL-8 production from DCs and macrophages. CCL20 regulate recruitment of Th17 cells to inflamed tissues. IL, interleukin; TH17, IL-17-producing T helper; ACT1, actin related gene 1; TRAF, TNF-receptor-associated factor; STAT3, signal transducers and activators of transcription 3; NF-κB, nuclear factor kappa B; MAPK, mitogen-activated protein kinase; EpC, epithelial cell; EC, endothelial cell; FB, fibroblast; MØ, macrophage; NK, natural killer; DC, dendritic cell; CCL, CC chemokine ligand; CCR6, CC chemokine receptor 6; JAK, janus kinase; MMPs, matrix metalloproteinases; NO, nitric oxide; GC, germinal center; Bcl-6, B-cell CLL/lymphoma 6; IgG, immunoglobulin G. |
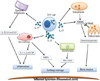 | Fig. 4The role of IL-17 in autoimmune arthritis. IL-17 leads to inflammation, cartilage damage and bone erosion in arthritis. In synovial fibroblast and macrophage, IL-17 induces pro-inflammatory cytokines such as IL-6, IL-8, TNF, IL-1β or MMPs. When acting on monocytes, IL-17 contributes to inflammation by increasing the production of pro-inflammatory cytokines. IL-17 activates the production and function of matrix metalloproteinases. IL-17 increases the expression of RANKL in osteoblasts, which leads to increased RANK signalling in osteoclasts. IL-17 produced by Th17 cells influences the activity of various cell types, and consequently leads to the development of features characteristic of rheumatoid arthritis including inflammation and destruction of cartilage and bone. IL, interleukin; Th17, IL-17-producing T helper; TNF, tumour necrosis factor; RANK, receptor activator NF kappa B; RANKL, RANK ligand; MMPs, matrix metalloproteinases. |
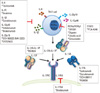 | Fig. 5Targeting of Th17 cells. There are several ways in which members of the IL-17 protein and receptor family can be targeted. Various therapeutic tools include blocking the differentiation and amplification of Th17 cells, inhibiting or neutralizing the cytokines of Th17 cells, and suppressing the transcription factors specific for Th17 cells. IL, interleukin; Th17, IL-17-producing T helper; STAT3, signal transducers and activators of transcription 3; ROR, retinoic-acid-receptor-related orphan receptor. |
References
1. Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000. 165:6107–6115.

2. Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005. 201:233–240.

3. Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005. 6:1133–1141.

4. Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007. 448:480–483.

5. Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007. 8:967–974.

6. Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006. 7:1151–1156.

7. McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009. 10:314–324.

8. Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012. 13:991–999.

9. Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010. 467:967–971.

10. Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008. 454:350–352.

11. Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008. 9:641–649.

12. Ichiyama K, Sekiya T, Inoue N, Tamiya T, Kashiwagi I, Kimura A, et al. Transcription factor Smad-independent T helper 17 cell induction by transforming-growth factor-beta is mediated by suppression of eomesodermin. Immunity. 2011. 34:741–754.

13. Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008. 453:106–109.

14. Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007. 204:1849–1861.

15. Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009. 106:4793–4798.
16. Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011. 12:255–263.

17. Lexberg MH, Taubner A, Albrecht I, Lepenies I, Richter A, Kamradt T, et al. IFN-gamma and IL-12 synergize to convert in vivo generated Th17 into Th1/Th17 cells. Eur J Immunol. 2010. 40:3017–3027.

18. Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY, et al. Control of TH17 cells occurs in the small intestine. Nature. 2011. 475:514–518.

19. Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009. 30:155–167.

20. Chang SH, Reynolds JM, Pappu BP, Chen G, Martinez GJ, Dong C. Interleukin-17C promotes Th17 cell responses and autoimmune disease via interleukin-17 receptor E. Immunity. 2011. 35:611–621.

21. Kleinschek MA, Owyang AM, Joyce-Shaikh B, Langrish CL, Chen Y, Gorman DM, et al. IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med. 2007. 204:161–170.

22. Ramirez-Carrozzi V, Sambandam A, Luis E, Lin Z, Jeet S, Lesch J, et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol. 2011. 12:1159–1166.

23. Hot A, Miossec P. Effects of interleukin (IL)-17A and IL-17F in human rheumatoid arthritis synoviocytes. Ann Rheum Dis. 2011. 70:727–732.

24. Shen F, Hu Z, Goswami J, Gaffen SL. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006. 281:24138–24148.

25. Shi P, Zhu S, Lin Y, Liu Y, Liu Y, Chen Z, et al. Persistent stimulation with interleukin-17 desensitizes cells through SCFbeta-TrCP-mediated degradation of Act1. Sci Signal. 2011. 4:ra73.
26. Zhu S, Pan W, Shi P, Gao H, Zhao F, Song X, et al. Modulation of experimental autoimmune encephalomyelitis through TRAF3-mediated suppression of interleukin 17 receptor signaling. J Exp Med. 2010. 207:2647–2662.

27. Zhong B, Liu X, Wang X, Chang SH, Liu X, Wang A, et al. Negative regulation of IL-17-mediated signaling and inflammation by the ubiquitin-specific protease USP25. Nat Immunol. 2012. 13:1110–1117.

28. Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006. 203:2673–2682.

29. Chabaud M, Miossec P. The combination of tumor necrosis factor alpha blockade with interleukin-1 and interleukin-17 blockade is more effective for controlling synovial inflammation and bone resorption in an ex vivo model. Arthritis Rheum. 2001. 44:1293–1303.

30. Ogura H, Murakami M, Okuyama Y, Tsuruoka M, Kitabayashi C, Kanamoto M, et al. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. 2008. 29:628–636.

31. Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev. 2008. 226:57–79.

32. Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJ, Joosten LA, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004. 50:650–659.

33. Koenders MI, Kolls JK, Oppers-Walgreen B, van den Bersselaar L, Joosten LA, Schurr JR, et al. Interleukin-17 receptor deficiency results in impaired synovial expression of interleukin-1 and matrix metalloproteinases 3, 9, and 13 and prevents cartilage destruction during chronic reactivated streptococcal cell wall-induced arthritis. Arthritis Rheum. 2005. 52:3239–3247.

34. Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet. 2009. 41:199–204.

35. Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007. 445:648–651.

36. Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002. 8:500–508.

37. Venken K, Hellings N, Hensen K, Rummens JL, Stinissen P. Memory CD4+CD127high T cells from patients with multiple sclerosis produce IL-17 in response to myelin antigens. J Neuroimmunol. 2010. 226:185–191.

38. Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006. 314:1461–1463.

39. Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med. 2009. 206:525–534.

40. Ito R, Kita M, Shin-Ya M, Kishida T, Urano A, Takada R, et al. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem Biophys Res Commun. 2008. 377:12–16.

41. Webb R, Merrill JT, Kelly JA, Sestak A, Kaufman KM, Langefeld CD, et al. A polymorphism within IL21R confers risk for systemic lupus erythematosus. Arthritis Rheum. 2009. 60:2402–2407.

42. Leng RX, Pan HF, Chen GM, Feng CC, Fan YG, Ye DQ, et al. The dual nature of Ets-1: focus to the pathogenesis of systemic lupus erythematosus. Autoimmun Rev. 2011. 10:439–443.

43. Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J Immunol. 2007. 178:3822–3830.

44. Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010. 2:52ra72.

45. Xu T, Wang X, Zhong B, Nurieva RI, Ding S, Dong C. Ursolic acid suppresses interleukin-17 (IL-17) production by selectively antagonizing the function of RORgamma t protein. J Biol Chem. 2011. 286:22707–22710.

46. Patel AM, Moreland LW. Interleukin-6 inhibition for treatment of rheumatoid arthritis: a review of tocilizumab therapy. Drug Des Devel Ther. 2010. 4:263–278.

47. Geyer M, Muller-Ladner U. Actual status of antiinterleukin-1 therapies in rheumatic diseases. Curr Opin Rheumatol. 2010. 22:246–251.

48. Gottlieb A, Menter A, Mendelsohn A, Shen YK, Li S, Guzzo C, et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet. 2009. 373:633–640.

49. Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008. 371:1665–1674.

50. Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008. 371:1675–1684.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download