Abstract
Purpose
Ischemia and reperfusion (I/R) injury is a major cause of hepatic failure after liver surgery, but there is no direct method to monitor it in real-time (like an ECG in heart disease) during surgery. Recently we found the possible role of bioelectrical impedance (BEI) to monitor I/R injury in liver, but the mechanism responsible for ischemia-related BEI changes has not been clearly determined.
Methods
The authors used a LCR meter to quantify BEI changes at 0.12 KHz. Livers were subjected to 70% partial ischemia for 120 minutes, and ATP content, cation changes in extracellular fluid (ECF; determined using an in vivo intracellular microdialysis technique), hepatocyte sizes, and histological changes were then examined.
Results
Liver tissue BEI was found to increase gradually during the first 60 minutes of ischemia and then tended to plateau. During the same period, intracellular ATP content decreased to below 20% of the baseline level, [Na+] in ECF decreased from 150.4±3.8 to 97.8±10.6 mmol/L, and [K+] in ECF increased from 7.5±0.3 to 34.3±5.5 mmol/L during the first 60 minutes of ischemia. Hepatocyte diameter increased by approximately 20% during the first 60 minutes of ischemia.
Figures and Tables
 | Fig. 1Changes of bioelectrical impedance in liver during ischemia according to frequency 120 Hz to 100 KHz (n=5). Ref. 20 with permission from the Korean Association of Hepato-Biliary and Pancreas Surgery. |
 | Fig. 2Change of bioelectrical impedance in liver during 30 minutes ischemia and 20 minutes reperfusion at 120 Hz. Bars represent means values±SD (n=5). Ref. 20 with permission from the Korean Association of Hepato-Biliary and Pancreas Surgery. |
 | Fig. 3Change of bioelectrical impedance in liver during 120 minutes ischemia. Bars represent means values±SD (n=5). Ref. 20 with permission from the Korean Association of Hepato-Biliary and Pancreas Surgery. |
 | Fig. 4ATP content of the ischemic liver at 30 minutes interval during 120 minutes ischemia. Ref. 22 with permission from Korean Surgical Society. |
 | Fig. 5Palmitic acid oxidation rate of the ischemic liver at 30 minutes interval during 120 minutes ischemia. Ref. 22 with permission from Korean Surgical Society. |
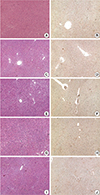 | Fig. 6Microscopic findings of the ischemic liver during 120 minutes ischemia. Ref. 22 with permission from Korean Surgical Society. (A, C, E, G, I) hematoxylin eosin (H&E) stain×40, (B, D, F, H, J) TUNEL stain×40. (A, B) control, (C, D) 30 minutes ischemia, (E, F) 60 minutes ischemia, (G, H) 90 minutes ischemia, (I, J) 120 minutes ischemia. |
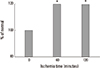 | Fig. 7Changes in hepatocyte diameter during 120 minutes of ischemia. *P<0.05 vs 0 minutes (non-ischemia). Ref. 14 with permission from Korean Surgical Society. |
 | Fig. 8Changes in ECF [Na+] and [K+] in liver tissues in vivo during 120 minutes of ischemia. *P<0.05 vs 0 minutes (non-ischemia). Ref. 14 with permission from Korean Surgical Society. |
 | Fig. 9The correlation between BEI and summed [Na+] and [K+] in liver tissue ECF in vivo. Ref. 14 with permission from Korean Surgical Society. |
References
2. Pelton JJ, Hoffman JP, Eisenberg BL. Comparison of liver function tests after hepatic lobectomy and hepatic wedge resection. Am Surg. 1998; 64:408–414.
3. Shoup M, Gonen M, D'Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003; 7:325–330.

4. Zoli M, Marchesini G, Melli A, Viti G, Marra A, Marrano D, et al. Evaluation of liver volume and liver function following hepatic resection in man. Liver. 1986; 6:286–291.

5. Imamura H, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg. 2005; 12:16–22.

6. Haemmerich D, Ozkan R, Tungjitkusolmun S, Tsai JZ, Mahvi DM, Staelin ST, et al. Changes in electrical resistivity of swine liver after occlusion and postmortem. Med Biol Eng Comput. 2002; 40:29–33.

7. Sasaki E, Hirose H, Ito H, Bando M, Senga S. Dielectric spectrogram for instantaneous evaluation of ischemic injury of the liver. ASAIO J. 1995; 41:M356–M359.

8. Harms J, Schneider A, Baumgartner M, Henke J, Busch R. Diagnosing acute liver graft rejection: experimental application of an implantable telemetric impedance device in native and transplanted porcine livers. Biosens Bioelectron. 2001; 16:169–177.

9. Gheorghiu M, Gersing E, Gheorghiu E. Quantitative analysis of impedance spectra of organs during ischemia. Ann N Y Acad Sci. 1999; 873:65–71.

10. Faes TJ, van der Meij HA, de Munck JC, Heethaar RM. The electric resistivity of human tissues (100 Hz-10 MHz): a meta-analysis of review studies. Physiol Meas. 1999; 20:R1–R10.

11. Khan HA. Bioluminometric assay of ATP in mouse brain: Determinant factors for enhanced test sensitivity. J Biosci. 2003; 28:379–382.

12. Kim JY, Koves TR, Yu GS, Gulick T, Cortright RN, Dohm GL, et al. Evidence of a malonyl-CoA-insensitive carnitine palmitoyltransferase I activity in red skeletal muscle. Am J Physiol Endocrinol Metab. 2002; 282:E1014–E1022.

13. Grubb BR, Chadburn JL, Boucher RC. In vivo microdialysis for determination of nasal liquid ion composition. Am J Physiol Cell Physiol. 2002; 282:C1423–C1431.

14. Cui ML, Ahn HS, Kim JY, Lee DS, Kim HJ, Yun SS. Impaired Cation Transport May Lead to Bioelectrical Impedance Changes during Hepatic Ischemia. J Korean Surg Soc. 2010; 78:390–397.

15. Champion HR, Jones RT, Trump BF, Decker R, Wilson S, Miginski M, et al. A clinicopathologic study of hepatic dysfunction following shock. Surg Gynecol Obstet. 1976; 142:657–663.

16. Kaiho T, Miyazaki M, Ito H, Ambiru S, Shimizu H, Togawa A, et al. Reduced hepatic functional reserve in cirrhosis and obstructive jaundice with special reference to histological morphometric analysis and galactose elimination capacity. Eur Surg Res. 1996; 28:333–340.

17. Nauta RJ, Tsimoyiannis E, Uribe M, Walsh DB, Miller D, Butterfield A. The role of calcium ions and calcium channel entry blockers in experimental ischemia-reperfusion-induced liver injury. Ann Surg. 1991; 213:137–142.

18. Grimnes S, Martinsen O. Bioimpedance and Bioelectricity Basics. New York: Academic Press;2000. p. 99–101.
19. Arthur C, Guyton M, Hall J. Textbook of medical physiology. Philadelphia: WB Saunders;2000. p. 874–875.
20. Cho YS, Yun SS, Shin HJ, Ahn HS, Lee DS, Kim HJ. Significance of bioelectrical impedance during ischemia-reperfusion injury in the rabbit's liver. Korean J Hepatobiliary Pancreat Surg. 2006; 10(1):29–33.
21. Cui ML, Ahn HS, Kim JY, Shin HJ, Lee DS, Kim HJ, et al. Bioelectrical impedance may predict cell viability during ischemia and reperfusion in rat liver. J Korean Med Sci. 2010; 25:577–582.

22. Park SH, Yun SS, Lee DS, Kim HJ, Choi JH, Kim JY. Estimation of liver cell viability after ischemia and reperfusion injury in rat liver. J Korean Surg Soc. 2007; 73:1–7.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download