Abstract
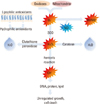
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are natural by-products of cellular physiological processes involving metabolism of compounds containing oxygen and nitrogen, respectively. Physiological defense mechanisms against ROS/RNS readily convert them into water or urea, but dysregulation of ROS/RNS production damages cells resulting in abnormal conditions such as uncontrolled growth or cell death. ROS/RNS are closely related to the development of a variety of diseases such as cancer, diabetes, neurodegeneration, vascular disease and chronic inflammation. Thus, it has been proposed that the removal of ROS/RNS may prevent or treat oxidative stress-induced diseases. Some antioxidant molecules are synthesized in the body, while others are obtained from food in the diet including fruits, vegetables, meat and even in natural water. In addition to the natural antioxidants, synthetic antioxidants have been modified from natural chemicals so as to increase bioavailability to target organs and increase stability in the air. In developing novel antioxidants for therapeutic use, some factors to consider are: 1) improved efficacy; 2) low side effects (comparatively clear mechanism); 3) competitive price and 4) improved convenience of dosing. In this review, we will discuss the issues mentioned above and the use of antioxidants in clinical application.
Figures and Tables
References
1. Bartz RR, Piantadosi CA. Clinical review: oxygen as a signaling molecule. Crit Care. 2010; 14:234.

3. Runchel C, Matsuzawa A, Ichijo H. Mitogen-activated protein kinases in mammalian oxidative stress responses. Antioxid Redox Signal. 2011; 15:205–218.

4. Pan JS, Hong MZ, Ren JL. Reactive oxygen species: a double-edged sword in oncogenesis. World J Gastroenterol. 2009; 15:1702–1707.

5. Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. J Biomed Biotechnol. 2012; 2012:936486.

6. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007; 39:44–84.

7. Devasagayam TP, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD. Free radicals and antioxidants in human health: current status and future prospects. J Assoc Physicians India. 2004; 52:794–804.
8. Ortega R. Importance of functional foods in the Mediterranean diet. Public Health Nutr. 2006; 9:1136–1140.

9. Arrigoni O, De Tullio MC. Ascorbic acid: much more than just an antioxidant. Biochim Biophys Acta. 2002; 1569:1–9.

10. Herrera E, Barbas C. Vitamin E: action, metabolism and perspectives. J Physiol Biochem. 2001; 57:43–56.

11. Mak JC. Potential role of green tea catechins in various disease therapies: progress and promise. Clin Exp Pharmacol Physiol. 2012; 39:265–273.

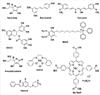
12. Ak T, Gulcin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. 2008; 174:27–37.

13. Chen WF, Deng SL, Zhou B, Yang L, Liu ZL. Curcumin and its analogues as potent inhibitors of low density lipoprotein oxidation: H-atom abstraction from the phenolic groups and possible involvement of the 4-hydroxy-3-methoxyphenyl groups. Free Radic Biol Med. 2006; 40:526–535.
14. Grynkiewicz G, Slifirski P. Curcumin and curcuminoids in quest for medicinal status. Acta Biochim Pol. 2012; 59:201–212.

15. Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992; 339:1523–1526.

16. Hung LM, Chen JK, Huang SS, Lee RS, Su MJ. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc Res. 2000; 47:549–555.

17. Kelkel M, Jacob C, Dicato M, Diederich M. Potential of the dietary antioxidants resveratrol and curcumin in prevention and treatment of hematologic malignancies. Molecules. 2010; 15:7035–7074.

18. Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, et al. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005; 280:17038–17045.

19. Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008; 8:157–168.

21. Jones DP, Carlson JL, Mody VC, Cai J, Lynn MJ, Sternberg P. Redox state of glutathione in human plasma. Free Radic Biol Med. 2000; 28:625–635.

22. Levy EJ, Anderson ME, Meister A. Transport of glutathione diethyl ester into human cells. Proc Natl Acad Sci U S A. 1993; 90:9171–9175.

23. Dringen R, Hamprecht B. N-acetylcysteine, but not methionine or 2-oxothiazolidine-4-carboxylate, serves as cysteine donor for the synthesis of glutathione in cultured neurons derived from embryonal rat brain. Neurosci Lett. 1999; 259:79–82.

24. Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral Nacetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med. 1988; 319:1557–1562.

25. Koch A, Trautwein C. N-acetylcysteine on its way to a broader application in patients with acute liver failure. Hepatology. 2010; 51:338–340.

26. Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, et al. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem. 2001; 276:4588–4596.

27. Smith RA, Porteous CM, Gane AM, Murphy MP. Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci U S A. 2003; 100:5407–5412.
28. Rodriguez-Cuenca S, Cocheme HM, Logan A, Abakumova I, Prime TA, Rose C, et al. Consequences of long-term oral administration of the mitochondria-targeted antioxidant MitoQ to wild-type mice. Free Radic Biol Med. 2010; 48:161–172.

29. Smith RA, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad Sci. 2010; 1201:96–103.

30. Doughan AK, Dikalov SI. Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid Redox Signal. 2007; 9:1825–1836.

31. Batinic-Haberle I, Reboucas JS, Spasojevic I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid Redox Signal. 2010; 13:877–918.

32. Shirinzadeh H, Eren B, Gurer-Orhan H, Suzen S, Ozden S. Novel indolebased analogs of melatonin: synthesis and in vitro antioxidant activity studies. Molecules. 2010; 15:2187–2202.

33. Kim HJ, Koo SY, Ahn BH, Park O, Park DH, Seo DO, et al. NecroX as a novel class of mitochondrial reactive oxygen species and ONOO(-) scavenger. Arch Pharm Res. 2010; 33:1813–1823.

34. Choi JM, Park KM, Kim SH, Hwang DW, Chon SH, Lee JH, et al. Effect of necrosis modulator necrox-7 on hepatic ischemia-reperfusion injury in beagle dogs. Transplant Proc. 2010; 42:3414–3421.

35. Park JH, Seo KS, Tadi S, Ahn BH, Lee JU, Heo JY, et al. An indole derivative protects against acetaminophen-induced liver injury by directly binding to N-Acetyl-p-Benzoquinone imine in mice. Antioxid Redox Signal. 2013; 18:1713–1722.

36. Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006; 28:219–242.

37. Spagnuolo C, Russo M, Bilotto S, Tedesco I, Laratta B, Russo GL. Dietary polyphenols in cancer prevention: the example of the flavonoid quercetin in leukemia. Ann N Y Acad Sci. 2012; 1259:95–103.

38. Cutando A, Lopez-Valverde A, Arias-Santiago S, J DEV, RG DED. Role of melatonin in cancer treatment. Anticancer Res. 2012; 32:2747–2753.
39. Warner DS, Sheng H, Batinic-Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol. 2004; 207:3221–3231.

40. Phases of clinical research [Internet]. Wikipedia. 2013. cited 2013 Mar 15. Available from: http://en.wikipedia.org/wiki/Phases_ of_clinical_research.
42. Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005; 294:56–65.

43. Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004; 164:2335–2342.
44. Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996; 334:1145–1149.

45. Milman U, Blum S, Shapira C, Aronson D, Miller-Lotan R, Anbinder Y, et al. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2-2 genotype: a prospective double-blinded clinical trial. Arterioscler Thromb Vasc Biol. 2008; 28:341–347.

46. Duffield-Lillico AJ, Reid ME, Turnbull BW, Combs GF Jr, Slate EH, Fischbach LA, et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002; 11:630–639.
47. Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007; 297:842–857.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download