Abstract
Reactive Oxygen Species (ROS) are a class of signaling molecules that regulate intracellular signaling cascades in response to external stimuli. Once accumulated in cells, they can damage DNA modifying gene transcription and affecting protein expression and function in ways that accelerate tumorigenesis. In cancer cells, the accumulation of ROS can increase cell proliferation and cell invasion into other tissues, while, antioxidant enzymes and molecules can protect cells from oxidative stress so as to maintain cellular homeostatic redox status. Cancer cells often do not have sufficient levels of antioxidant enzymes which are needed to rescue cells from oxidative stress. The redox status of cancer cells appears to be a key factor in maintaining the malignant phenotype. Cancer stem cells, on the other hand, have been shown to maintain low levels of ROS in order to retain their self renewal and differentiation potential, even though the exact mechanism is not known yet. ROS and antioxidant enzymes are novel targets for developing anti-cancer therapeutics. In this review, the current understanding for redox regulation of cancer cells and neoplastic stem cells as well as the role and function of anti-oxidant enzymes and molecules is discussed.
Figures and Tables
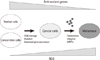
Fig. 1
Regulation of reactive oxygen species and anti-oxidant genes during tumorigenesis. During tumorigenesis from normal cells and cancer stem cells, ROS is accumulated, but the expression of anti-oxidant genes is reduced. Accumulated ROS induces DNA damage, mutation and abnormal gene expression, leading to transformation into cancer cells.
ACKNOWLEDGMENT
This work was supported in part by grants from the GRL project and the New Drug Target Discovery Project (M10848000352-08N4800-35210), the Ministry of Education, Science & Technology, Republic of Korea.
References
1. Bystrom LM, Guzman ML, Rivella S. Iron and Reactive Oxygen Species: Friends or Foes of Cancer Cells? Antioxid Redox Siganl. 2013; in press.

2. Jena NR. DNA damage by reactive species: Mechanisms, mutation and repair. J Biosci. 2012; 37:503–517.

3. Mates JM, Segura JA, Alonso FJ, Marquez J. Oxidative stress in apoptosis and cancer: an update. Arch Toxicol. 2012; 86:1649–1665.

4. Tochhawng L, Deng S, Pervaiz S, Yap CT. Redox regulation of cancer cell migration and invasion. Mitochondrion. 2013; 13:246–253.

5. Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012; 5:9–19.

6. Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, et al. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. 1997; 272:217–221.
7. Junn E, Lee KN, Ju HR, Han SH, Im JY, Kang HS, et al. Requirement of hydrogen peroxide generation in TGF-beta 1 signal transduction in human lung fibroblast cells: involvement of hydrogen peroxide and Ca2+ in TGF-beta 1-induced IL-6 expression. J Immunol. 2000; 165:2190–2197.

8. Cui X. Reactive oxygen species: the achilles' heel of cancer cells? Antioxid Redox Signal. 2012; 16:1212–1214.

10. Curtin NJ. DNA repair dysregulation from cancer driver to therapeutic target. Nat Rev Cancer. 2012; 12:801–817.

11. Kotsinas A, Aggarwal V, Tan EJ, Levy B, Gorgoulis VG. PIG3: a novel link between oxidative stress and DNA damage response in cancer. Cancer Lett. 2012; 327:97–102.

12. Maillet A, Pervaiz S. Redox regulation of p53, redox effectors regulated by p53: a subtle balance. Antioxid Redox Signal. 2012; 16:1285–1294.

13. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010; 49:1603–1616.

14. Kobayashi CI, Suda T. Regulation of reactive oxygen species in stem cells and cancer stem cells. J Cell Physiol. 2012; 227:421–430.

15. Shi X, Zhang Y, Zheng J, Pan J. Reactive oxygen species in cancer stem cells. Antioxid Redox Signal. 2012; 16:1215–1228.

16. Dhar SK, Tangpong J, Chaiswing L, Oberley TD, St Clair DK. Manganese superoxide dismutase is a p53-regulated gene that switches cancers between early and advanced stages. Cancer Res. 2011; 71:6684–6695.

17. Lee S, Kim SM, Lee RT. Thioredoxin and thioredoxin target proteins: from molecular mechanisms to functional significance. Antioxid Redox Signal. 2013; 18:1165–1207.

18. Nyström T, Yang J, Molin M. Peroxiredoxins, gerontogenes linking aging to genome instability and cancer. Genes Dev. 2012; 26:2001–2008.

19. Shin D, Jeon JH, Jeong M, Suh HW, Kim S, Kim HC, et al. VDUP1 mediates nuclear export of HIF1alpha via CRM1-dependent pathway. Biochim Biophys Acta. 2008; 1783:838–848.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download