Abstract
Reactive oxygen species (ROS) are harmful to cellular components such as proteins, DNA and lipids. The continuous production of ROS during the respiratory electron transfer process has been regarded as the major cause of aging. However, the discoveries of proteins whose structure and function switch with cellular ROS suggest that ROS are active players in cellular regulation. OxyR is the first protein whose ROS-regulated mechanism was revealed by the atomic structure studies. The distantly-located two cysteines in OxyR form a disulfide bond by reaction with ROS, resulting in conformational and functional switches in the protein. The heat shock protein 33 is another protein that is activated by increased level of cellular ROS. Many other cellular proteins including protein tyrosine phosphatases are also regulated by ROS. This review focuses on the structure and function of the ROS-regulated proteins and their implications on the ROS's cellular roles. Detailed studies on the ROS-generating protein machinery and the ROS-regulated proteins should contribute to the therapeutic control of ROS-related diseases and aging processes.
Figures and Tables
 | Fig. 1ROS's cellular function regulation network. The mitochondria-derived ROS play two roles including cellular function regulation and promotion of diseases/aging. The cellular function regulation by ROS is likely to feedback its results to mitochondria, resulting in the control of ROS generation level. It will be necessary to understand the balance between the ROS's effects on diseases/aging and the cellular function regulation. |
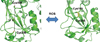 | Fig. 2ROS-mediated structural switch in OxyR. The reduced OxyR (left-hand side) has two cysteines (Cys199 and Cys208) that are distant to each other (the inter-sulfur atom distance: 17 Å). ROS oxidize the two cysteines, resulting in a disulfide bond of the oxidized OxyR (right-hand side). For the disulfide bond formation, two cysteines travel to a close location to each other, causing large structural transitions that affect the monomeric and dimeric interaction surfaces and promote the oxidized OxyR-mediated transcription of antioxidant proteins. |
References
3. Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012; 48:158–167.

4. Ryu SE. Structural mechanism of disulphide bond-mediated redox switches. J Biochem. 2012; 151:579–588.

5. Choi H, Kim S, Mukhopadhyay P, Cho S, Woo J, Storz G, et al. Structural basis of the redox switch in the OxyR transcription factor. Cell. 2001; 105:103–113.

6. Lee C, Lee SM, Mukhopadhyay P, Kim SJ, Lee SC, Ahn WS, et al. Redox regulation of OxyR requires specific disulfide bond formation involving a rapid kinetic reaction path. Nat Struct Mol Biol. 2004; 11:1179–1185.

7. Mishra P, Socolich M, Wall MA, Graves J, Wang Z, Ranganathan R. Dynamic scaffolding in a G protein-coupled signaling system. Cell. 2007; 131:80–92.

8. Liu W, Wen W, Wei Z, Yu J, Ye F, Liu CH, et al. The INAD scaffold is a dynamic, redox-regulated modulator of signaling in the Drosophila eye. Cell. 2011; 145:1088–1101.

10. Zhou A, Carrell RW, Murphy MP, Wei Z, Yan Y, Stanley PL, et al. A redox switch in angiotensinogen modulates angiotensin release. Nature. 2010; 468:108–111.

11. Hoffmann DS, Weydert CJ, Lazartigues E, Kutschke WJ, Kienzle MF, Leach JE, et al. Chronic tempol prevents hypertension, proteinuria, and poor feto-placental outcomes in BPH/5 mouse model of preeclampsia. Hypertension. 2008; 51:1058–1065.

12. Yoo SK, Starnes TW, Deng Q, Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature. 2011; 480:109–112.

13. Reece SY, Nocera DG. Proton-coupled electron transfer in biology: results from synergistic studies in natural and model systems. Annu Rev Biochem. 2009; 78:673–699.

14. Koopman WJ, Nijtmans LG, Dieteren CE, Roestenberg P, Valsecchi F, Smeitink JA, et al. Mammalian mitochondrial complex I: biogenesis, regulation, and reactive oxygen species generation. Antioxid Redox Signal. 2010; 12:1431–1470.

15. Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol. 2010; 45:466–472.

16. Marchi S, Giorgi C, Suski JM, Agnoletto C, Bononi A, Bonora M, et al. Mitochondria-ros crosstalk in the control of cell death and aging. J Signal Transduct. 2012; 2012:329635.

17. Dudkina NV, Kudryashev M, Stahlberg H, Boekema EJ. Interaction of complexes I, III, and IV within the bovine respirasome by single particle cryoelectron tomography. Proc Natl Acad Sci U S A. 2011; 108:15196–15200.

18. Althoff T, Mills DJ, Popot JL, Kuhlbrandt W. Arrangement of electron transport chain components in bovine mitochondrial supercomplex I1III2IV1. EMBO J. 2011; 30:4652–4664.

19. Block K, Gorin Y. Aiding and abetting roles of NOX oxidases in cellular transformation. Nat Rev Cancer. 2012; 12:627–637.

20. Pallas M, Verdaguer E, Tajes M, Gutierrez-Cuesta J, Camins A. Modulation of sirtuins: new targets for antiageing. Recent Pat CNS Drug Discov. 2008; 3:61–69.

21. Chung JS, Park S, Park SH, Park ER, Cha PH, Kim BY, et al. Overexpression of Romo1 promotes production of reactive oxygen species and invasiveness of hepatic tumor cells. Gastroenterology. 2012; 143:1084–1094.e7.

22. Budanov AV. Stress-responsive sestrins link p53 with redox regulation and mammalian target of rapamycin signaling. Antioxid Redox Signal. 2011; 15:1679–1690.

23. Gertz M, Steegborn C. The Lifespan-regulator p66Shc in mitochondria: redox enzyme or redox sensor? Antioxid Redox Signal. 2010; 13:1417–1428.

24. Wang X, Mukhopadhyay P, Wood MJ, Outten FW, Opdyke JA, Storz G. Mutational analysis to define an activating region on the redox-sensitive transcriptional regulator OxyR. J Bacteriol. 2006; 188:8335–8342.

25. Kim SO, Merchant K, Nudelman R, Beyer WF Jr, Keng T, DeAngelo J, et al. OxyR: a molecular code for redox-related signaling. Cell. 2002; 109:383–396.
26. Kim SJ, Jeong DG, Chi SW, Lee JS, Ryu SE. Crystal structure of proteolytic fragments of the redox-sensitive Hsp33 with constitutive chaperone activity. Nat Struct Biol. 2001; 8:459–466.
27. Graumann J, Lilie H, Tang X, Tucker KA, Hoffmann JH, Vijayalakshmi J, et al. Activation of the redox-regulated molecular chaperone Hsp33--a two-step mechanism. Structure. 2001; 9:377–387.

28. Reichmann D, Xu Y, Cremers CM, Ilbert M, Mittelman R, Fitzgerald MC, et al. Order out of disorder: working cycle of an intrinsically unfolded chaperone. Cell. 2012; 148:947–957.

29. Vijayalakshmi J, Mukhergee MK, Graumann J, Jakob U, Saper MA. The 2.2 A crystal structure of Hsp33: a heat shock protein with redox-regulated chaperone activity. Structure. 2001; 9:367–375.
30. Chi SW, Jeong DG, Woo JR, Lee HS, Park BC, Kim BY, et al. Crystal structure of constitutively monomeric E. coli Hsp33 mutant with chaperone activity. FEBS Lett. 2011; 585:664–670.

31. Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell. 2005; 121:667–670.

32. Kwon J, Lee SR, Yang KS, Ahn Y, Kim YJ, Stadtman ER, et al. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci U S A. 2004; 101:16419–16424.

34. Jeong DG, Kim SJ, Kim JH, Son JH, Park MR, Lim SM, et al. Trimeric structure of PRL-1 phosphatase reveals an active enzyme conformation and regulation mechanisms. J Mol Biol. 2005; 345:401–413.

35. van Montfort RL, Congreve M, Tisi D, Carr R, Jhoti H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature. 2003; 423:773–777.

36. Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, et al. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003; 423:769–773.

37. Yang J, Groen A, Lemeer S, Jans A, Slijper M, Roe SM, et al. Reversible oxidation of the membrane distal domain of receptor PTPalpha is mediated by a cyclic sulfenamide. Biochemistry. 2007; 46:709–719.

38. Choi HJ, Kang SW, Yang CH, Rhee SG, Ryu SE. Crystal structure of a novel human peroxidase enzyme at 2.0 A resolution. Nat Struct Biol. 1998; 5:400–406.
39. Haque A, Andersen JN, Salmeen A, Barford D, Tonks NK. Conformation-sensing antibodies stabilize the oxidized form of PTP1B and inhibit its phosphatase activity. Cell. 2011; 147:185–198.

40. Park H, Park SY, Oh JJ, Ryu SE. Identification of potent VHZ phosphatase inhibitors with structure-based virtual screening. J Biomol Screen. 2013; 18:226–231.

41. Park H, Chien PN, Ryu SE. Discovery of potent inhibitors of receptor protein tyrosine phosphatase sigma through the structure-based virtual screening. Bioorg Med Chem Lett. 2012; 22:6333–6337.

42. Sarkis M, Tran DN, Kolb S, Miteva MA, Villoutreix BO, Garbay C, et al. Design and synthesis of novel bis-thiazolone derivatives as micromolar CDC25 phosphatase inhibitors: effect of dimerisation on phosphatase inhibition. Bioorg Med Chem Lett. 2012; 22:7345–7350.

43. Galasko DR, Peskind E, Clark CM, Quinn JF, Ringman JM, Jicha GA, et al. Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Arch Neurol. 2012; 69:836–841.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download