Abstract
Follicular helper T cells (Tfh) play a significant role in providing T cell help to B cells during the germinal center reaction, where somatic hypermutation, affinity maturation, isotype class switching, and the differentiation of memory B cells and long-lived plasma cells occur. Antigen-specific T cells with IL-6 and IL-21 upregulate CXCR5, which is required for the migration of T cells into B cell follicles, where these T cells mature into Tfh. The surface markers including PD-1, ICOS, and CD40L play a significant role in providing T cell help to B cells. The upregulation of transcription factor Bcl-6 induces the expression of CXCR5, which is an important factor for Tfh differentiation, by inhibiting the expression of other lineage-specific transcription factors such as T-bet, GATA3, and RORγt. Surprisingly, recent evidence suggests that CD4 T cells already committed to Th1, Th2, and Th17 cells obtain flexibility in their differentiation programs by downregulating T-bet, GATA3, and RORγt, upregulating Bcl-6 and thus convert into Tfh. Limiting the numbers of Tfh within germinal centers is important in the regulation of the autoantibody production that is central to autoimmune diseases. Recently, it was revealed that the germinal center reaction and the size of the Tfh population are also regulated by thymus-derived follicular regulatory T cells (Tfr) expressing CXCR5 and Foxp3. Dysregulation of Tfh appears to be a pathogenic cause of autoimmune disease suggesting that tight regulation of Tfh and germinal center reaction by Tfr is essential for maintaining immune tolerance. Therefore, the balance between Tfh and Tfr appears to be a critical peripheral tolerance mechanism that can inhibit autoimmune disorders.
Figures and Tables
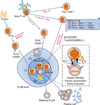 | Fig. 1Germinal center formation controlled by Tfh and Tfr. Naïve T cells interact with Ag-presenting DC and differentiate into specific helper T cell subsets upon TCR stimulation under specific cytokine environment. IL-6 and IL-21 activate Bcl-6 expression which determines T cells fate to Tfh by expression of CXCR5. Tfh migrates to B cell area via CXCL13-CXCR5 interaction, and they contribute germinal center (GC) formation with follicular dendritic cells (fDC). Tfh provides T cell help to GC B cell induces isotype class-switching, somatic hypermutation, and affinity maturation via IL-21 and IL-4. For GC formation, interaction between Tfh and B cells essentially mediate through ICOS-ICOSL and CD40-CD40L. GC B cell will differentiate into plasma cells and memory B cells which is necessary for humoral immune responses. Tfh-mediated GC formation is regulated by thymic nTreg-derived Tfr cells expressing Foxp3 and CXCR5. Tfr migrate to B cell area to control Tfh and GC B cell responses which regulate GC formation and humoral response to inhibit autoimmune diseases. |
ACKNOWLEDGEMENTS
This work is supported by Basic Science Research Program through National Research Foundation of Korea grants (2012-0003073 and 2012-0007210). The authors declare no competing financial interests.
References
1. King C, Tangye SG, Mackay CR. T follicular helper (Tfh) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008. 26:741–766.

2. Velardi A, Mingari MC, Moretta L, Grossi CE. Functional analysis of cloned germinal center CD4+ cells with natural killer cell-related features. Divergence from typical T helper cells. J Immunol. 1986. 137:2808–2813.
3. Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000. 192:1545–1552.

4. Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000. 192:1553–1562.

5. Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991. 354:389–392.

6. Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991. 67:1121–1129.

7. Liu YJ, Malisan F, de Bouteiller O, Guret C, Lebecque S, Banchereau J, et al. Within germinal centers, isotype switching of immunoglobulin genes occurs after the onset of somatic mutation. Immunity. 1996. 4:241–250.

9. Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998. 16:111–135.

10. Dong C, Temann UA, Flavell RA. Cutting edge: critical role of inducible costimulator in germinal center reactions. J Immunol. 2001. 166:3659–3662.

11. Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009. 325:1006–1010.

12. Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009. 325:1001–1005.

13. Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009. 31:457–468.

14. Nakayamada S, Takahashi H, Kanno Y, O'Shea JJ. Helper T cell diversity and plasticity. Curr Opin Immunol. 2012. 24:297–302.

15. Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One. 2011. 6:e17739.

16. Diehl SA, Schmidlin H, Nagasawa M, Blom B, Spits H. IL-6 Triggers IL-21 production by human CD4(+) T cells to drive STAT3-dependent plasma cell differentiation in B cells. Immunol Cell Biol. 2012. 90:802–811.

17. Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004. 173:5361–5371.

18. Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science. 1997. 276:589–592.

19. Basso K, Saito M, Sumazin P, Margolin AA, Wang K, Lim WK, et al. Integrated biochemical and computational approach identifies BCL6 direct target genes controlling multiple pathways in normal germinal center B cells. Blood. 2010. 115:975–984.

20. Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. 2010. 185:313–326.

21. Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007. 179:5099–5108.

22. Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004. 173:68–78.

23. Oestreich KJ, Mohn SE, Weinmann AS. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a Tfh-like gene profile. Nat Immunol. 2012. 13:405–411.

24. Kim JI, Ho IC, Grusby MJ, Glimcher LH. The transcription factor c-Maf controls the production of interleukin-4 but not other Th2 cytokines. Immunity. 1999. 10:745–751.

25. Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009. 10:167–175.

26. Yusuf I, Kageyama R, Monticelli L, Johnston RJ, Ditoro D, Hansen K, et al. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150). J Immunol. 2010. 185:190–202.

27. Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002. 298:1630–1634.

28. Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005. 175:7867–7879.

29. Kuchen S, Robbins R, Sims GP, Sheng C, Phillips TM, Lipsky PE, et al. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J Immunol. 2007. 179:5886–5896.

30. Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of Tfh cell differentiation. J Exp Med. 2012. 209:243–250.

31. Bossaller L, Burger J, Draeger R, Grimbacher B, Knoth R, Plebani A, et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J Immunol. 2006. 177:4927–4932.

32. Ono M, Shimizu J, Miyachi Y, Sakaguchi S. Control of autoimmune myocarditis and multiorgan inflammation by glucocorticoid-induced TNF receptor family-related protein(high), Foxp3-expressing CD25+ and CD25- regulatory T cells. J Immunol. 2006. 176:4748–4756.

33. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003. 299:1057–1061.

34. Wollenberg I, Agua-Doce A, Hernandez A, Almeida C, Oliveira VG, Faro J, et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol. 2011. 187:4553–4560.

35. Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011. 17:983–988.

36. Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011. 17:975–982.

37. Vinuesa CG, Cook MC. The molecular basis of lymphoid architecture and B cell responses: implications for immunodeficiency and immunopathology. Curr Mol Med. 2001. 1:689–725.

38. Luzina IG, Atamas SP, Storrer CE, daSilva LC, Kelsoe G, Papadimitriou JC, et al. Spontaneous formation of germinal centers in autoimmune mice. J Leukoc Biol. 2001. 70:578–584.
39. Victoratos P, Kollias G. Induction of autoantibody-mediated spontaneous arthritis critically depends on follicular dendritic cells. Immunity. 2009. 30:130–142.

40. Daikh DI, Finck BK, Linsley PS, Hollenbaugh D, Wofsy D. Long-term inhibition of murine lupus by brief simultaneous blockade of the B7/CD28 and CD40/gp39 costimulation pathways. J Immunol. 1997. 159:3104–3108.
41. Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005. 435:452–458.

42. Duffau P, Seneschal J, Nicco C, Richez C, Lazaro E, Douchet I, et al. Platelet CD154 potentiates interferon-alpha secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci Transl Med. 2010. 2:47ra63.
43. Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009. 206:561–576.

44. Bubier JA, Sproule TJ, Foreman O, Spolski R, Shaffer DJ, Morse HC 3rd, et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Natl Acad Sci U S A. 2009. 106:1518–1523.

45. Steiner G, Smolen J. Autoantibodies in rheumatoid arthritis and their clinical significance. Arthritis Res. 2002. 4:Suppl 2. S1–S5.
46. Mohan C, Shi Y, Laman JD, Datta SK. Interaction between CD40 and its ligand gp39 in the development of murine lupus nephritis. J Immunol. 1995. 154:1470–1480.
47. Grammer AC, Slota R, Fischer R, Gur H, Girschick H, Yarboro C, et al. Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154-CD40 interactions. J Clin Invest. 2003. 112:1506–1520.

48. Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008. 205:2873–2886.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download