Abstract
Decades of accumulated knowledge and improved comprehension of various perspectives on rheumatoid arthritis (RA) pathophysiology has led to the development of new biologic agents that inhibit a specific component of the RA inflammatory process. Especially during the last two decades, several epochal agents which target tumor necrosis factor-α, interleukin-1, interleukin-6, CD20-expressing B cell, and cytotoxic T lymphocyte antigen-4 were used in the management of RA and other autoimmune diseases with highly comparable efficacy and safety. Moreover, dozens of innovative agents queue up for clinical trials day by day. Herein, we review the current scenario of RA management in terms of pathogenesis and targeted molecular pathways, and some important controversies in this field as well. Based on the complications that these kinds of diseases pose, it is highly reasonable to hope that further improved therapies and more tailored drugs for RA will be introduced in the near future.
Figures and Tables
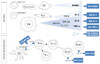 | Fig. 1Targets of biologic agent in rheumatoid arthritis. OC, osteoclast; DC, dendritic cell; FLS, fibroblast-likesynoviocyte; Mφ, macrophage; RANKL, Receptor activator of nuclear factor kappa B ligand; TGF-β, Transforming growth factor beta; TNF-α, Tumor necrosis factor alpha; Syk, Spleen tyrosine kinase; Tyk, Tyrosine kinase; CC, β-chemokine; CXC, α-Chemokine; GM-CSF, Granulocyte macrophage-colony stimulating factor; Th, T helper IL, Interleukin; CD, Cluster of differentiation. |
References
1. Schenkier S, Golbus J. Treatment of rheumatoid arthritis. New thoughts on the classic pyramid approach. Postgrad Med. 1992. 91:285–286. 289–292.
2. Wilske KR. Inverting the therapeutic pyramid: observations and recommendations on new directions in rheumatoid arthritis therapy based on the author's experience. Semin Arthritis Rheum. 1993. 23:11–18.

3. Buch MH, Emery P. New therapies in the management of rheumatoid arthritis. Curr Opin Rheumatol. 2011. 23:245–251.

4. van Vollenhoven RF. Treatment of rheumatoid arthritis: state of the art 2009. Nat Rev Rheumatol. 2009. 5:531–541.

7. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011. 365:2205–2219.

8. Rigby WF. Drug insight: different mechanisms of action of tumor necrosis factor antagonists-passive-aggressive behavior? Nat Clin Pract Rheumatol. 2007. 3:227–233.

9. Knight DM, Trinh H, Le J, Siegel S, Shealy D, McDonough M, et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol. 1993. 30:1443–1453.

10. Kornbluth A. Infliximab approved for use in Crohn's disease: a report on the FDA GI Advisory Committee conference. Inflamm Bowel Dis. 1998. 4:328–329.

11. Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al. ATTRACT Study Group. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. Lancet. 1999. 354:1932–1939.

12. Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000. 343:1594–1602.

13. Etanercept. Soluble tumour necrosis factor receptor, TNF receptor fusion protein, TNFR-Fc, TNR 001, Enbrel. Drugs R D. 1999. 1:258–261.
14. Klareskog L, van der Heijde D, de Jager JP, Gough A, Kalden J, Malaise M, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004. 363:675–681.

15. van der Heijde D, Klareskog L, Singh A, Tornero J, Melo-Gomes J, Codreanu C, et al. Patient reported outcomes in a trial of combination therapy with etanercept and methotrexate for rheumatoid arthritis: the TEMPO trial. Ann Rheum Dis. 2006. 65:328–334.

16. van Riel PL, Freundlich B, MacPeek D, Pedersen R, Foehl JR, Singh A. Patient-reported health outcomes in a trial of etanercept monotherapy versus combination therapy with etanercept and methotrexate for rheumatoid arthritis: the ADORE trial. Ann Rheum Dis. 2008. 67:1104–1110.

17. Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003. 48:35–45.

18. Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006. 54:26–37.

19. Keystone E, Heijde D, Mason D Jr, Landewe R, Vollenhoven RV, Combe B, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 2008. 58:3319–3329.

20. Keystone EC, Genovese MC, Klareskog L, Hsia EC, Hall ST, Miranda PC, et al. Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Ann Rheum Dis. 2009. 68:789–796.

21. Smolen JS, Kay J, Doyle MK, Landewe R, Matteson EL, Wollenhaupt J, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009. 374:210–221.

22. Cohen S, Hurd E, Cush J, Schiff M, Weinblatt ME, Moreland LW, et al. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002. 46:614–624.

23. Furst DE, Keystone EC, Kirkham B, Kavanaugh A, Fleischmann R, Mease P, et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2008. Ann Rheum Dis. 2008. 67:Suppl 3. iii2–iii25.
24. McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007. 7:429–442.

25. Maini RN, Taylor PC, Szechinski J, Pavelka K, Broll J, Balint G, et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006. 54:2817–2829.

26. Radin AR, Mellis SJ, Jasson M, Nadler D, Belomestnov P, Wu R, et al. REGN88/SAR153191, a fully-human interleukin-6 receptor monoclonal antibody, reduces acute phase reactants in patients with rheumatoid arthritis: preliminary observations from phase 1 studies. Arthritis Rheum. 2010. 62:Suppl 10. 1121.
27. Mease P, Strand V, Shalamberdize L. Inhibition of IL-6 with ALD518 improves disease activity in rheumatoid arthritis in a randomized, double-blind, placebo-controlled, dose ranging phase 2 clinical trial. Ann Rheum Dis. 2010. 69:98.
28. Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010. 2:52ra72.

29. Rheumatology 2010 annual scientific meeting. Secukinumab (AIN457), a novel monoclonal antibody targeting IL-17A demonstrates efficacy in active rheumatoid arthritis patients despite stable methotrexate treatment: results of a phase IIb study. Arthritis Rheum. 2010. 62:L9.
30. Gizinski AM, Fox DA, Sarkar S. Pharmacotherapy: concepts of pathogenesis and emerging treatments. Co-stimulation and T cells as therapeutic targets. Best Pract Res Clin Rheumatol. 2010. 24:463–477.
31. Westhovens R, Robles M, Ximenes AC, Nayiager S, Wollenhaupt J, Durez P, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009. 68:1870–1877.

32. Bathon J, Robles M, Ximenes AC, Nayiager S, Wollenhaupt J, Durez P, et al. Sustained disease remission and inhibition of radiographic progression in methotrexate-naive patients with rheumatoid arthritis and poor prognostic factors treated with abatacept: 2-year outcomes. Ann Rheum Dis. 2011. 70:1949–1956.

33. Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2006. 144:865–876.

34. Schiff M, Pritchard C, Huffstutter JE, Rodriguez-Valverde V, Durez P, Zhou X, et al. The 6-month safety and efficacy of abatacept in patients with rheumatoid arthritis who underwent a washout after anti-tumour necrosis factor therapy or were directly switched to abatacept: the ARRIVE trial. Ann Rheum Dis. 2009. 68:1708–1714.

35. Cohen SB. Targeting the B cell in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2010. 24:553–563.

36. Edwards JC, Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol. 2006. 6:394–403.

37. Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004. 350:2572–2581.

38. Cohen SB, Keystone E, Genovese MC, Emery P, Peterfy C, Tak PP, et al. Continued inhibition of structural damage over 2 years in patients with rheumatoid arthritis treated with rituximab in combination with methotrexate. Ann Rheum Dis. 2010. 69:1158–1161.

39. Genovese MC, Kaine JL, Lowenstein MB, Del Giudice J, Baldassare A, Schechtman J, et al. Ocrelizumab, a humanized anti-CD20 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I/II randomized, blinded, placebo-controlled, dose-ranging study. Arthritis Rheum. 2008. 58:2652–2661.

40. Taylor PC, Quattrocchi E, Mallett S, Kurrasch R, Petersen J, Chang DJ. Ofatumumab, a fully human anti-CD20 monoclonal antibody, in biological-naive, rheumatoid arthritis patients with an inadequate response to methotrexate: a randomised, double-blind, placebo-controlled clinical trial. Ann Rheum Dis. 2011. 70:2119–2125.

41. Calero I, Nieto JA, Sanz I. B cell therapies for rheumatoid arthritis: beyond B cell depletion. Rheum Dis Clin North Am. 2010. 36:325–343.

42. Tak PP, Thurlings RM, Rossier C, Nestorov I, Dimic A, Mircetic V, et al. Atacicept in patients with rheumatoid arthritis: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating, single- and repeated-dose study. Arthritis Rheum. 2008. 58:61–72.

43. Cha HS, Boyle DL, Inoue T, Schoot R, Tak PP, Pine P, et al. A novel spleen tyrosine kinase inhibitor blocks c-Jun N-terminal kinase-mediated gene expression in synoviocytes. J Pharmacol Exp Ther. 2006. 317:571–578.

44. Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009. 60:1895–1905.

45. Cohen SB, Dore RK, Lane NE, Ory PA, Peterfy CG, Sharp JT, et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheu matoidarthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 2008. 58:1299–1309.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download