Abstract
Chronic pathological pain is sustained by mechanisms of peripheral and central sensitization, which are being increasingly investigated at the molecular and cellular levels. The molecular mechanisms of sensitization that occur in peripheral nociceptors and the dorsal horns of the spinal cord are putative targets for context-dependent drugs. Pregabalin and gabapentin are analogs of the neurotransmitter gamma-aminobutyric acid (GABA). They are alpha2-delta ligands that have analgesic, anticonvulsant, and anxiolytic activity. Alpha2-delta is an auxiliary protein associated with voltage-gated calcium channels. They bind potently to the subunit resulting in modulation of calcium channels and reduction in the release of several neurotransmitters. This review discusses the pharmacology of these medications briefly as well as available clinical applications in pain management.
Figures and Tables
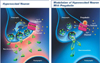 | Fig. 1The mechanism of action of pregabalin. Pregabalin modulates hyperexcited neurons via the following mechanism: Pregabalin binds to presynaptic neurons at the alpha2-delta (α 2-δ) subunit of voltage-gated calcium channels. Drug binding reduces calcium influx into presynaptic terminals. Decreased calcium influx reduces excessive release of excitatory neurotransmitters (eg, glutamate, substance P, noradrenaline). Data on file, Pfizer Inc, New York, NY, USA. |
References
1. Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000. 16:521–555.

2. Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005. 57:411–425.

3. Hofmann F, Biel M, Flockerzi V. Molecular basis for Ca2+ channel diversity. Annu Rev Neurosci. 1994. 17:399–418.

4. Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, Catterall WA. Nomenclature of voltage-gated calcium channels. Neuron. 2000. 25:533–535.

5. Chaplan SR, Pogrel JW, Yaksh TL. Role of voltage-dependent calcium channel subtypes in experimental tactile allodynia. J Pharmacol Exp Ther. 1994. 269:1117–1123.
6. Malmberg AB, Yaksh TL. Voltage-sensitive calcium channels in spinal nociceptive processing: blockade of N- and P-type channels inhibits formalin-induced nociception. J Neurosci. 1994. 14:4882–4890.

7. Bowersox SS, Gadbois T, Singh T, Pettus M, Wang YX, Luther RR. Selective N-type neuronal voltage-sensitive calcium channel blocker, SNX-111, produces spinal antinociception in rat models of acute, persistent and neuropathic pain. J Pharmacol Exp Ther. 1996. 279:1243–1249.
8. Neugebauer V, Vanegas H, Nebe J, Rumenapp P, Schaible HG. Effects of N- and L-type calcium channel antagonists on the responses of nociceptive spinal cord neurons to mechanical stimulation of the normal and the inflamed knee joint. J Neurophysiol. 1996. 76:3740–3749.

9. Diaz A, Dickenson AH. Blockade of spinal N- and P-type, but not L-type, calcium channels inhibits the excitability of rat dorsal horn neurones produced by subcutaneous formalin inflammation. Pain. 1997. 69:93–100.

10. Sluka KA. Blockade of N- and P/Q-type calcium channels reduces the secondary heat hyperalgesia induced by acute inflammation. J Pharmacol Exp Ther. 1998. 287:232–237.
11. Jay SD, Sharp AH, Kahl SD, Vedvick TS, Harpold MM, Campbell KP. Structural characterization of the dihydropyridine-sensitive calcium channel alpha 2-subunit and the associated delta peptides. J Biol Chem. 1991. 266:3287–3293.

12. Marais E, Klugbauer N, Hofmann F. Calcium channel alpha(2)delta subunits-structure and Gabapentin binding. Mol Pharmacol. 2001. 59:1243–1248.

13. Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996. 271:5768–5776.

14. Wang M, Offord J, Oxender DL, Su TZ. Structural requirement of the calcium-channel subunit alpha2delta for gabapentin binding. Biochem J. 1999. 342:313–320.

15. Bian F, Li Z, Offord J, Davis MD, McCormick J, Taylor CP, Walker LC. Calcium channel alpha2-delta type 1 subunit is the major binding protein for pregabalin in neocortex, hippocampus, amygdala, and spinal cord: an ex vivo autoradiographic study in alpha2-delta type 1 genetically modified mice. Brain Res. 2006. 1075:68–80.

16. Li CY, Song YH, Higuera ES, Luo ZD. Spinal dorsal horn calcium channel alpha2delta-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia. J Neurosci. 2004. 24:8494–8499.

17. Melrose HL, Kinloch RA, Cox PJ, Field MJ, Collins D, Williams D. [3H] pregabalin binding is increased in ipsilateral dorsal horn following chronic constriction injury. Neurosci Lett. 2007. 417:187–192.

18. Luo ZD, Calcutt NA, Higuera ES, Valder CR, Song YH, Svensson CI, Myers RR. Injury type-specific calcium channel alpha 2 delta-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J Pharmacol Exp Ther. 2002. 303:1199–1205.

19. Guay DR. Pregabalin in neuropathic pain: a more "pharmaceutically elegant" gabapentin? Am J Geriatr Pharmacother. 2005. 3:274–287.

20. Micheva KD, Taylor CP, Smith SJ. Pregabalin reduces the release of synaptic vesicles from cultured hippocampal neurons. Mol Pharmacol. 2006. 70:467–476.

21. Maneuf YP, Gonzalez MI, Sutton KS, Chung FZ, Pinnock RD, Lee K. Cellular and molecular action of the putative GABA-mimetic, gabapentin. Cell Mol Life Sci. 2003. 60:742–750.

22. Taylor CP, Gee NS, Su TZ, Kocsis JD, Welty DF, Brown JP, Dooley DJ, Boden P, Singh L. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res. 1998. 29:233–249.

23. Silverman RB, Andruszkiewicz R, Nanavati SM, Taylor CP, Vartanian MG. 3-Alkyl-4-aminobutyric acids: the first class of anticonvulsant agents that activates L-glutamic acid decarboxylase. J Med Chem. 1991. 34:2295–2298.

24. Tassone DM, Boyce E, Guyer J, Nuzum D. Pregabalin: a novel gamma-aminobutyric acid analogue in the treatment of neuropathic pain, partial-onset seizures, and anxiety disorders. Clin Ther. 2007. 29:26–48.

25. Gilron I. Gabapentin and pregabalin for chronic neuropathic and early postsurgical pain: current evidence and future directions. Curr Opin Anaesthesiol. 2007. 20:456–472.

26. Backonja M, Glanzman RL. Gabapentin dosing for neuropathic pain: evidence from randomized, placebo-controlled clinical trials. Clin Ther. 2003. 25:81–104.

27. Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005. 352:1324–1334.

28. Victorri-Vigneau C, Guerlais M, Jolliet P. Abuse, dependency and withdrawal with gabapentin: a first case report. Pharmacopsychiatry. 2007. 40:43–44.

29. Grosshans M, Mutschler J, Hermann D, Klein O, Dressing H, Kiefer F, Mann K. Pregabalin abuse, dependence, and withdrawal: a case report. Am J Psychiatry. 2010. 167:869.

30. Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002. 18:350–354.

31. van Seventer R, Feister HA, Young JP Jr, Stoker M, Versavel M, Rigaudy L. Efficacy and tolerability of twice-daily pregabalin for treating pain and related sleep interference in postherpetic neuralgia: a 13-week, randomized trial. Curr Med Res Opin. 2006. 22:375–384.

32. Chiechio S, Zammataro M, Caraci F, Rampello L, Copani A, Sabato AF, Nicoletti F. Pregabalin in the treatment of chronic pain: an overview. Clin Drug Investig. 2009. 29:203–213.
33. Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004. 110:628–638.

34. Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004. 63:2104–2110.

35. Sabatowski R, Galvez R, Cherry DA, Jacquot F, Vincent E, Maisonobe P, Versavel M. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trial. Pain. 2004. 109:26–35.

36. Bandelow B, Wedekind D, Leon T. Pregabalin for the treatment of generalized anxiety disorder: a novel pharmacologic intervention. Expert Rev Neurother. 2007. 7:769–781.

37. Skyler JS. Diabetic complications. The importance of glucose control. Endocrinol Metab Clin North Am. 1996. 5:243–254.
38. Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990. 33:160–172.

39. Mease PJ, Clauw DJ, Arnold LM, Goldenberg DL, Witter J, Williams DA, Simon LS, Strand CV, Bramson C, Martin S, Wright TM, Littman B, Wernicke JF, Gendreau RM, Crofford LJ. Fibromyalgia syndrome. J Rheumatol. 2005. 32:2270–2277.
40. Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995. 38:19–28.

41. Tzellos TG, Toulis KA, Goulis DG, Papazisis G, Zampeli VA, Vakfari A, Kouvelas D. Gabapentin and pregabalin in the treatment of fibromyalgia: a systematic review and a meta-analysis. J Clin Pharm Ther. 2010. 35:639–656.

43. Crofford LJ, Mease PJ, Simpson SL, Young JP Jr, Martin SA, Haig GM, Sharma U. Fibromyalgia relapse evaluation and efficacy for durability of meaningful relief (FREEDOM): a 6-month, double-blind, placebo-controlled trial with pregabalin. Pain. 2008. 136:419–431.

44. Tzellos TG, Papazisis G, Toulis KA, Sardeli C, Kouvelas D. A2delta ligands gabapentin and pregabalin: future implications in daily clinical practice. Hippokratia. 2010. 4:71–75.
45. Gray P. Pregabalin in the management of central neuropathic pain. Expert Opin Pharmacother. 2007. 8:3035–3041.

46. Siddall PJ, Cousins MJ, Otte A, Griesing T, Chambers R, Murphy TK. Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology. 2006. 67:1792–1800.

47. Vondracek P, Oslejskova H, Kepak T, Mazanek P, Sterba J, Rysava M, Gal P. Efficacy of pregabalin in neuropathic pain in paediatric oncological patients. Eur J Paediatr Neurol. 2009. 13:332–336.

48. Hening W, Allen RP, Tenzer P, Winkelman JW. Restless legs syndrome: demographics, presentation, and differential diagnosis. Geriatrics. 2007. 62:26–29.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download