Abstract
Orofacial neuropathic pain is initiated by extraction of teeth or nerve injury from trauma in the trigeminal nerve that innervates the facial area. In the experiment, orofacial neuropathic pain usually occurred following injury of peripheral trigeminal nerve including infra-orbital nerve, inferior alveolar nerve, or mental nerve. In addition, pathology from trigeminal nerve root or ganglion is involved in orofacial neuropathic pain. This study introduced various animal models that help us study the underlying mechanisms of development or maintenance of orofacial neuropathic pain. One of the most typical symptoms of orofacial neuropathic pain is hypersensitivity to the innocuous mechanical stimuli. Our study presents a novel method to evaluate mechanical allodynia in rats with orofacial neuropathic pain. Recently, accumulate evidence support participation of central glial cells in the development or maintenance of orofacial neuropathic pain. Signaling molecules in glial cells also play an important role in neuropathic pain in the orofacial area.
Figures and Tables
Fig. 1
Illustration of various animal models for orofacial neuropathic pain. (A) Branches of the trigeminal nerve; infraorbital nerve, inferior alveolar nerve, mental nerve. (B) ION-CCI model. Infraorbital nerve was exposed and separated from adhering tissue and two ligatures were made around it. Arrow indicates infraorbital nerve.
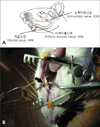
Fig. 2
Time course in the ipsilateral and contralateral airpuff thresholds following ION-CCI in the rat. *p<0.05, shamvs. ION-CCI-treated group. Original date presented in Lim et al (2007).

Fig. 3
Images showing the animal model for malpositioned dental implant-induced neuropathic pain. The dental implant was placed in the alveolar socket after extraction of lower second molar tooth (A). The inferior alveolar canal was penetrated by incorrect placement of dental implant (B).

Fig. 4
Time course analysis of the changes in air-puff thresholds following incorrectly placed dental implants in rats. Treatment with mal-positioned dental implants produced significant mechanical allodynia in both ipsilateral (A) and contralateral sides (B). *p<0.05, malpositioned dental implant (MDI) vs. sham group. Original date presented in Han et al.(2010).

Fig. 5
Illustration of a trigeminal neuralgia animal model produced by compression of the trigeminal ganglion.
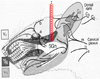
Fig. 6
Change in the a amount of pressure following application of air-puff (A) and von Frey filament (B). Both showed proportionate relationship between the amount of pressure and intensity of stimulation.
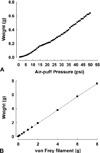
Fig. 7
Typical responses of a neuron to air-puff or Von Frey stimulation. The neuron (WDR) responded to brush (A), von Frey filament (B), air-puff (C), and pinch (D) stimulation. Neuronal firings increased by air puff and von Frey filament stimulation (E).
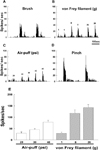
Fig. 8
The effects of an intracisternal injection of PD98059, a MEK inhibitor (A) and SB203580, a p38 MAPK inhibitor (B) on ION-CCI-induced mechanical allodynia in the orofacial area. P<0.05, the vehicle- vs. chemicalstreated group. #P<0.05, 1 µg of chemicals-treated group vs. 10 µg of chemicals-treated group. Original date presented in Lim et al.(2007).

References
2. Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988. 33:87–107.

3. Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990. 43:205–218.

4. Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992. 50:355–363.

5. Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000. 87:149–158.

6. Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rats infraorbital nerve. J Neurosci. 1994. 14:2708–2723.

7. Lim EJ, Jeon HJ, Yang GY, Lee MK, Ju JS, Han SR, Ahn DK. Intracisternal administration of mitogen-activated protein kinase inhibitors reduced mechanical allodynia following chronic constriction injury of infraorbital nerve in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2007. 31:1322–1329.

8. Han SR, Yeo SP, Lee MK, Bae YC, Ahn DK. Early dexamethasone relieves trigeminal neuropathic pain. J Dent Res. 2010. 89:915–920.

9. Jannetta PJ. Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg. 1967. 26:159–162.

10. Love S, Coakham HB. Trigeminal neuralgia: pathology and pathogenesis. Brain. 2001. 124:2347–2360.

11. Ahn DK, Lim EJ, Kim BC, Yang GY, Lee MK, Ju JS, Han SR, Bae YC. Compression of the trigeminal ganglion produces prolonged nociceptive behavior in rats. Eur J Pain. 2009. 13:568–575.

12. Ahn DK, Jung CY, Lee HJ, Choi HS, Ju JS, Bae YC. Peripheral glutamate receptors participate in interleukin-1beta-induced mechanical allodynia in the orofacial area of rats. Neurosci Lett. 2004. 357:203–206.

13. Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988. 32:77–88.

14. Ahn DK, Lee SY, Han SR, Ju JS, Yang GY, Lee MK, Youn DH, Bae YC. Intratrigeminal ganglionic injection of LPA causes neuropathic pain-like behavior and demyelination in rats. Pain. 2009. 146:114–120.

15. Park CK, Kim K, Jung SJ, Kim MJ, Ahn DK, Hong SD, Kim JS, Oh SB. Molecular mechanism for local anesthetic action of eugenol in the rat trigeminal system. Pain. 2009. 144:84–94.

16. Gosselin RD, Suter MR, Ji RR, Decosterd I. Glial cells and chronic pain. Neuroscientist. 2010. 16:519–531.

17. Beggs S, Salter MW. Stereological and somatotopic analysis of the spinal microglial response to peripheral nerve injury. Brain Behav Immun. 2007. 21:624–633.

18. Chang YW, Tan A, Saab C, Waxman S. Unilateral focal burn injury is followed by long-lasting bilateral allodynia and neuronal hyperexcitability in spinal cord dorsal horn. J Pain. 2010. 11:119–130.

19. Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in "small" glia. Trends Neurosci. 2005. 28:101–107.

20. Ji RR, Gereau RW 4th, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009. 60:135–148.

21. Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a threekinase module from yeast to human. Physiol Rev. 1999. 79:143–180.

22. Impey S, Obrietan K, Storm DR. Making new connections: role of ERK/MAP kinase signaling in neuronal plasticity. Neuron. 1999. 23:11–14.
23. White FA, Jung H, Miller RJ. Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci U S A. 2007. 104:20151–20158.

24. Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001. 8:1–10.

26. Terayama R, Fujisawa N, Yamaguchi D, Omura S, Ichikawa H, Sugimoto T. Differential activation of mitogen-activated protein kinases and glial cells in the trigeminal sensory nuclear complex following lingual nerve injury. Neurosci Res. 2011. 69:100–110.

27. Mei XP, Zhang H, Wang W, Wei YY, Zhai MZ, Xu LX, Li YQ. Inhibition of spinal astrocytic c-Jun N-terminal kinase (JNK) activation correlates with the analgesic effects of ketamine in neuropathic pain. J Neuroinflammation. 2011. 8:6.

28. Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, Tokunaga A, Noguchi K. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J Neurosci. 2004. 24:10211–10222.

29. Kommers T, Vinade L, Pereira C, Goncalves CA, Wofchuk S, Rodnight R. Regulation of the phosphorylation of glial fibrillary acidic protein (GFAP) by glutamate and calcium ions in slices of immature rat spinal cord: comparison with immature hippocampus. Neurosci Lett. 1998. 248:141–143.

30. Inoue A, Ikoma K, Morioka N, Kumagai K, Hashimoto T, Hide I, Nakata Y. Interleukin-1beta induces sub--stance P release from primary afferent neurons through the cyclooxygenase-2 system. J Neurochem. 1999. 73:2206–2213.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download