Abstract
Clinician must consider several factors when advising a breastfeeding mother on the compatibility of her medications to breastfeeding infants: the benefit the medication will give to the mother; the risk of discontinuation of breastfeeding to the baby, the risk of medication to the baby and the risk of medication to the maternal milk supply. In almost all situations, there are numerous medications that can be safely used for specific symtoms and should be carefully chosen with the breastfeeding mother in mind. The transfer of medication from the maternal serum into milk depends on the drug's oral availability, lipid solubility, molecular weight, protein binding and half life. One must remember when the mother uses medication, that medication needs to be absorbed into the bloodstream of the mother, be able to cross into milk, be orally available to the infant, absorbed by infant's GI tract, be able to get into the infant's bloodstream and be at an infant dose which is generally very small.
Figures and Tables
Fig. 2
Typical mammary alveolar subunit with the milk-producing alveolar cells (lactocytes) arrayed on thinner surfaces of the alveoli and surrounded by capillaries and adipose cells.(From Hale TW. Pharmacology review: drug therapy and breastfeeding: pharmacokinetics, risk factors and effects on milk production. NeoReviews 2004;5:e164-e172. Hale TW. Pharmacology review: drug therapy and breastfeeding: antibiotics, analgesics, and other medications. NeoReviews 2005;6:e233-e240.)
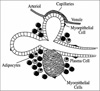
Fig. 3
A) Concentration-time profile for citalopram in human plasma and milk. Different but parallel concentrations in two compartments.(From 3. Rampono J, Kristensen JH, Hackett, LP, Paech M, Kohan R, Ilett KF. Citalopram and demethylcitalopram in human milk; distribution, excretion and effects in breast fed infants. Br J Clin Pharmacol 2000;50:263-68) B) Concentration-time profile for metformin in human plasma and milk following administration of 500 mg × 3/day.(From Hale TW, Kristensen JH, Hackett LP, Kohan R, Ilett KF. Transfer of metformin into human milk. Diabetologia 2002;45:1509-14.)

References
1. Hale TW. Pharmacology review: drug therapy and breastfeeding: pharmacokinetics, risk factors and effects on milk production. Neoreviews. 2004. 5:e164–e172.
2. Hale TW. Pharmacology review: drug therapy and breastfeeding: antibiotics, analgesics, and other medications. Neoreviews. 2005. 6:e233–e240.
3. Rampono J, Kristensen JH, Hackett LP, Paech M, Kohan R, Ilett KF. Citalopram and demethylcitalopram in human milk; distribution, excretion and effects in breast fed infants. Br J Clin Pharmacol. 2000. 50:263–268.

4. Hale TW, Kristensen JH, Hackett LP, Kohan R, Ilett KF. Transfer of metformin into human milk. Diabetologia. 2002. 45:1509–1514.

5. Rampono J, Kristensen JH, Kohan R, Page-Sharp M, Ilett KF. Transfer of escitalopram and its metabolite demethylescitalopram into breastmilk. Br J Clin Pharmacol. 2006. 62:316.

6. Lawrence RA, Lawrence RM. Breastfeeding, a guide for the medical profession. 2005. Philadelphia, USA: Elsevier Mosby.
7. Auerbach KG. Breastfeeding and maternal medication use. J Obstet Gynecol Neonatal Nurs. 1999. 28:554–563.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download