Abstract
To produce milk, four secretory processes are synchronized in the alveolar cell of the mature, functional mammary gland: (1) exocytosis, (2) fat synthesis and secretion, (3) secretion of ions and water, and (4) transcytosis of immunoglubulins and other substances from the interstitial space. Milk is synthesized continuously into the alveolar lumen, where it is stored until milk removal from the breast is initiated. Prolactin mediates the central nervous system regulation of milk secretion, but its influence is modified greatly by local factors that depend on milk removal from the breast. Oxytocin mediates milk let-down by stimulating the contraction of myoepithelial cells that surround the alveoli and ducts. Lactogenesis includes all the processes necessary to go from the undifferentiated mammary gland in the early pregnant animal to full lactation sometime after parturition. The most important factors in initiation of lactogenesis stage II appear to be progesterone withdrawal. The metabolic demands of breastfeeding require an increase in maternal metabolism. Postpartum suppression of fertility is thought to be the result of an alteration in pulsatile gonadotropin releasing hormone secretion from the hypothalamus. Women who wish to ensure against pregnancy during lactation usually are advised to use other contraceptive means.
Figures and Tables
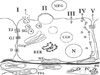 | Fig. 1The pathways for milk synthesis and secretion by the mammary epithelial cell. I=Exocytosis of milk protein, lactose, and other components of the aqueous phase in Golgi-derived secretory vesicles. II=Milk fact secretion by way of the milk fat globule. III=Direct movement of monovalent ions, water, and glucose across the apical membrane of the cell. IV=Transcytosis of components of the interstitial space. V=The paracellular pathway for plasma components and leukocytes. Pathway V is open only during pregnancy, involution, and in inflammatory states such as mastitis. Abbreviations : SG, secretory granule; RER, rough endoplasmic reticulum; BM, basement membrane; MFG, milk fat globule; CLD, cytoplasmic lipid droplet; N, nucleus; PC, plasma cell; FDA, fat depleted adipocyte; TJ, tight junction; GJ, gap junction; D, desmosome; ME, myoepithelial cell. (From Neville MC. Anatomy and physiology of lactation. Pediatr Clin North Am 2001;48:13-34.) |
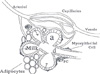 | Fig. 2Mammary alveolus. Milk is secreted into alveoli (a). The ductile (d) through which the milk is ejected by contraction of the myoepithelial cells is surrounded by supporting structures that include vasculature and a rich stroma composed of fibroblasts and adipocytes, and plasma cells (PC). (From Neville MC. Anatomy and physiology of lactation. Pediatr Clin North Am 2001;48:13-34.) |
References
3. Neville MC, Peaker M. Ionized calcium in milk and the integrity of the mammary epithelium in the goat. J Physiol. 1981. 313:561–570.

4. Neville MC. Jensen RG, editor. Volume and caloric density of human milk. Handbook of milk composition. 1995. San Diego, USA: Academic Press;101–113.

5. Butte NF, Villalpando S, Wong WW, Flores-Huerta S, Hernandez-Beltran MJ, Smith EO, Garza C. Human milk intake and growth faltering of rural Mesoamerindian infants. Am J Clin Nutr. 1992. 55:1109–1116.

7. Madden JD, Boyar RM, MacDonald PC, Porter JC. Analysis of secretory patterns of prolactin and gonadotropins during twenty-four hours in a lactating woman before and after resumption of menses. Am J Obstet Gynecol. 1978. 132:436–441.

8. Rigg LA, Lein A, Yen SS. Pattern of increase in circulating prolactin levels during human gestation. Am J Obstet Gynecol. 1977. 129:454–456.

9. Martin RH, Glass MR, Chapman C, Wilson GD, Woods KL. Human alpha-lactalbumin and hormonal factors in pregnancy and lactation. Clin Endocrinol (Oxf). 1980. 13:223–230.
10. Howie PW, McNeilly AS, McArdle T, Smart L, Houston M. The relationship between suckling-induced prolactin response and lactogenesis. J Clin Endocrinol Metab. 1980. 50:670–673.

11. Tyson JE. Crosignani PG, Robyn C, editors. Nursing and prolactin secretion: Principal determinants in the mediation of puerperal infertility. Prolactin and human reproduction. 1977. New York, USA: Academic Press;97–108.
12. Gross BA, Eastman CJ, Bowen KM, McElduff A. Integrated concentrations of prolactin in breast-feeding mothers. Aust N Z J Obstet Gynaecol. 1979. 19:150–153.

13. McNeilly AS, Tay CC, Glasier A. Physiological mechanisms underlying lactational amenorrhea. Ann N Y Acad Sci. 1994. 709:145–155.

14. Peaker M, Wilde CJ. Feedback control of milk secretion from milk. J Mammary Gland Biol Neoplasia. 1996. 1:307–315.

15. Millar ID, Barber MC, Lomax MA, Travers MT, Shennan DB. Mammary protein synthesis is acutely regulated by the cellular hydration state. Biochem Biophys Res Commun. 1997. 230:351–355.

16. Sudlow AW, Burgoyne RD. A hypo-osmotically induced increase in intracellular Ca2+ in lactating mouse mammary epithelial cells involving Ca2+ influx. Pflugers Arch. 1997. 433:609–616.

17. Crowley WR, Armstrong WE. Neurochemical regulation of oxytocin secretion in lactation. Endocr Rev. 1992. 13:33–65.

18. Cobo E, De Bernal MM, Gaitan E, Quintero CA. Neurohypophyseal hormone release in the human. II. Experimental study during lactation. Am J Obstet Gynecol. 1967. 97:519–529.
19. Ueda T, Yokoyama Y, Irahara M, Aono T. Influence of psychological stress on suckling-induced pulsatile oxytocin release. Obstet Gynecol. 1994. 84:259–262.
20. McNeilly AS, Robinson IC, Houston MJ, Howie PW. Release of oxytocin and prolactin in response to suckling. Br Med J (Clin Res Ed). 1983. 286:257–259.

21. Die Milchdrüse Dabelow A. Möllendorff WV, editor. Mikroskopischen Anatomie des Menschen. 1957. Berlin: Springer-Verlag;277.
23. Cobo E. Effect of different doses of ethanol on the milkejecting reflex in lactating women. Am J Obstet Gynecol. 1973. 115:817–821.

24. Coiro V, Alboni A, Gramellini D, Cigarini C, Bianconi L, Pignatti D, Volpi R, Chiodera P. Inhibition by ethanol of the oxytocin response to breast stimulation in normal women and the role of endogenous opioids. Acta Endocrinol. 1992. 126:213–216.

25. Haldar J, Sawyer WH. Inhibition of oxytocin release by morphine and its analogs. Proc Soc Exp Biol Med. 1978. 157:476–480.

26. Rayner VC, Robinson IC, Russell JA. Chronic intracerebroventricular morphine and lactation in rats: Dependence and tolerance in relation to oxytocin neurones. J Physiol. 1988. 396:319–347.

27. Pedersen CA, Prange AJ. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci U S A. 1979. 76:6661–6665.

28. Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci. 1994. 108:1163–1171.

29. Keverne EB, Kendrick KM. Maternal behavior in sheep and its neuroendocrine regulation. Acta Paediatr Suppl. 1994. 397:47–56.
30. Altemus M, Deuster PA, Galliven E, Carter CS, Gold PW. Suppression of hypothalmic-pituitary-adrenal axis responses to stress in lactating women. J Clin Endocrinol Metab. 1995. 80:2954–2959.

31. Chiodera P, Salvarani C, Bacchi-Modena A, Spallanzani R, Cigarini C, Alboni A, Gardini E, Coiro V. Relationship between plasma profiles of oxytocin and adrenocorticotropic hormone during suckling or breast stimulation in women. Horm Res. 1991. 35:119–123.

32. Arthur PG, Kent JC, Potter JM, Hartmann PE. Lactose in blood in nonpregnant, pregnant, and lactating women. J Pediatr Gastroenterol Nutr. 1991. 13:254–259.

33. Kuhn NJ. Peaker M, editor. Lactogenesis. The search for trigger mechanisms in different species. Comparative aspects of lactation. 1977. London: Academic Press;165.
35. Neville MC, Keller R, Seacat J, Lutes V, Neifert M, Casey C, Allen J, Archer P. Studies in human lactation: milk volumes in lactating women during the onset of lactation and full lactation. Am J Clin Nutr. 1988. 48:1375–1386.

36. Prentice AM, Whitehead RG. Loudon A, Racey T, editors. The energetics of human reproduction. Reproductive energetics in mammals. 1987. Oxford University Press: Oxford;275–304.
37. Cross NA, Hillman LS, Allen SH, Krause GF. Changes in bone mineral density and markers of bone remodeling during lactation and postweaning in women consuming high amounts of calcium. J Bone Miner Res. 1995. 10:1312–1320.

38. Krebs NF, Reidinger CJ, Robertson AD, Brenner M. Bone mineral density changes during lactation: maternal, dietary, and biochemical correlates. Am J Clin Nutr. 1997. 65:1738–1746.

39. Kalkwarf HJ. Hormonal and dietary regulation of changes in bone density during lactation and after weaning in women. J Mammary Gland Biol Neoplasia. 1999. 4:319–329.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download