Abstract
Objective
The aim of this study was to identify the expression of nitric oxide synthases (NOS) in the mandibular condyle during mandible advancement by functional appliance and to correlate it with the histologic changes and bone remodeling.
Methods
Twenty-four female, 35-day-old Sprague-Dawley rats were randomly divided into 3 experimental groups. In all experimental groups, the mandibles of the rats were kept in a continuous forward position with a fixed bite jumping appliance. The rats were sacrificed on the 3rd, 14th, and 30th days of experiment. More than 2 rats in each group were used for staining.
Results
There were no remarkable histologic changes and NOS expression differences in the control group. The most prominent histologic changes occurred in the 14th day experimental group. NOS decreased in the 30th day experimental group. There was increased expression of NOS2 and NOS3 in all experimental groups, comparative to the control group. In all the experimental groups and control group, the expression of NOS2 was greater than that of NOS3.
Figures and Tables
Fig. 2
Diagram of the mode of action of the bite-jumping appliance. Gray circle indicates the bite-jumping appliance. A, Normal position; B, forward position.

Fig. 3
Comparison of histologic changes in the condyle between the control group and experimental groups according to the appliance wearing time (H&E stain, × 40). A, Control group -3rd day with control condyle showing fibrous articular (A), proliferative (P), hypertrophic cartilage (H) zone and subchondral bone (S); B, experimental group -3rd day; C, control group -14th day showing a similar pattern to the 3rd day control group, with a well preserved layer of fibrous articular; D, experimental group -14th day; E, control group -30th day; F, experimental group -30th day. The 30 day control group (E) shows a similar pattern to the 14 day control group (C). The 30 day experiment group (F) shows similar patterns to the 3 day experiment group (B).
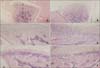
Fig. 4
Microphotography of the control and experimental groups at 3 days which were immunohistochemically stained for NOS2 and NOS3 (A, C, NOS2; B, D, NOS3, × 200). The expressions of NOS2 and NOS3 were rare in the condyles of the 3 day control group (expressions of NOS2 or NOS3 are indicated by arrow). The expression of NOS2 in the condyle (C) was more prominent in the 3 day experimental group than that of the control group (A), especially in the hypertrophic and proliferating zones (C). But the expression of NOS3 (D) was similar to that of the control group (B).
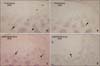
Fig. 5
Microphotography of the control and experimental groups at 14 days which were immunohistochemically stained for NOS2 and NOS3 (A, C, NOS2; B, D; NOS3, × 200). Expressions of NOS2 and NOS3 in subchondral bone of the condyle were noted (A, B). Both NOS2 and NOS3 expressions in the 14 days experimental group (C, D) were greater in the hypertrophic zone and subchondral bone than those of the control groups (A, B).

Fig. 6
Microphotography of the control and experimental group at 30 days which were immunohistochemically stained for NOS2 and NOS3 (A, C, NOS2; B, D, NOS3, × 200). The expressions of NOS2 and NOS3 in the condyle (A, B) were similar to those of control groups of 3 days and 14 days (Fig 4A, 4B, 5A, 5B). There were no significant differences between the expressions of NOS2 and NOS3 in the condyle of experimental group (C, D) relative to those of the control groups (A, B).

References
1. Ruf S, Pancherz H. Temporomandibular joint remodeling in adolescents and young adults during Herbst treatment: A prospective longitudinal magnetic resonance imaging and cephalometric radiographic investigation. Am J Orthod Dentofacial Orthop. 1999. 115:607–618.

2. McNamara JA Jr. Neuromuscular and skeletal adaptations to altered function in the orofacial region. Am J Orthod. 1973. 64:578–606.

3. Pancherz H. The Herbst appliance--its biologic effects and clinical use. Am J Orthod. 1985. 87:1–20.

4. Rabie AB, Zhao Z, Shen G, Hägg EU, Dr O, Robinson W. Osteogenesis in the glenoid fossa in response to mandibular advancement. Am J Orthod Dentofacial Orthop. 2001. 119:390–400.

5. McNamara JA Jr, Carlson DS. Quantitative analysis of temporomandibular joint adaptations to protrusive function. Am J Orthod. 1979. 76:593–611.

6. McNamara JA Jr, Bryan FA. Long-term mandibular adaptations to protrusive function: an experimental study in Macaca mulatta. Am J Orthod Dentofacial Orthop. 1987. 92:98–108.

7. Zaman G, Pitsillides AA, Rawlinson SC, Suswillo RF, Mosley JR, Cheng MZ, et al. Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J Bone Miner Res. 1999. 14:1123–1131.

8. Binderman I, Shimshoni Z, Somjen D. Biochemical pathways involved in the translation of physical stimulus into biological message. Calcif Tissue Int. 1984. 36:suppl 1. S82–S85.

9. Yeh CK, Rodan GA. Tensile forces enhance prostaglandin E synthesis in osteoblastic cells grown on collagen ribbons. Calif Tissue Int. 1984. 36:suppl 1. S67–S71.

10. Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985. 227:1477–1479.

11. Reich KM, Mcallister TN, Gudi S, Frangos JA. Activation of G proteins mediates flow-induced prostaglandin E2 production in osteoblasts. Endocrinology. 1997. 138:1014–1018.

12. Kuchan MJ, Frangos JA. Role of calcium and calmodulin in flow-induced nitric oxide production in endothelial cells. Am J Physiol. 1994. 266:C628–C636.

14. Morgan L. Nitric oxide: a challenge to chiropractic. J Can Chiropr Assoc. 2000. 44:40–48.
15. Fox SW, Chambers TJ, Chow JW. Nitric oxide is an early mediator of the increase in bone formation by mechanical stimulation. Am J Physiol. 1996. 270:E955–E960.

16. Turner CH, Takano Y, Owan I, Murrell GA. Nitric oxide inhibitor L-NAME suppresses mechanically induced bone formation in rats. Am J Physiol. 1996. 270:E634–E639.

17. Lamas S, Marsden PA, Li GK, Tempst P, Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A. 1992. 89:6348–6352.

18. Xie QW, Cho HJ, Calaycay J, Mumford RA, Swiderek KM, Lee TD, et al. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992. 256:225–228.

19. Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987. 84:9265–9269.

20. Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987. 327:524–526.

21. Villars F, Bordenave L, Bareille R, Amédée J. Effect of human endothelial cells on human bone marrow stromal cell phenotype: role of VEGF? J cell Biochem. 2000. 79:672–685.

22. Ferrara N. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int. 1999. 56:794–814.

23. Shum L, Rabie AB, Hägg U. Vascular endothelial growth factor expression and bone formation in posterior glenoid fossa during stepwise mandibular advancement. Am J Orthod Dentofacial Orthop. 2004. 125:185–190.

24. Rabie AB, Wong L, Hägg U. Correlation of replicating cells and osteogenesis in the glenoid fossa during stepwise advancement. Am J Orthod Dentofacial Orthop. 2003. 123:521–526.

25. Griffith OW, Stuehr DJ. Nitric oxide synthases: properties and catalytic mechanism. Annu Rev Physiol. 1995. 57:707–736.

26. Reif DW, McCreedy SA. N-nitro-L-arginine and N-monomethyl-L-arginine exhibit a different pattern of inactivation toward the three nitric oxide synthases. Arch Biochem Biophys. 1995. 320:170–176.

27. Pitsillides AA, Rawlinson SC, Suswillo RF, Bourrin S, Zaman G, Lanyon LE. Mechanical strain-induced NO production by bone cells: a possible role in adaptive bone (re)modeling? FASEB J. 1995. 9:1614–1622.

28. Woodside DG, Metaxas A, Altuna G. The influence of functional appliance therapy on glenoid fossa remodelling. Am J Orthod Dentofacial Orthop. 1987. 92:181–198.

29. Vargervik K, Harvold EP. Response to activator treatment in Class II malocclusions. Am J Orthod. 1985. 88:242–251.

30. Graber TM, Vanasdall RL Jr. Orthodontics, current principles and techniques. 2000. 3rd ed. St Louis: Mosby;473–520.
31. Hukkanen M, Hughes FJ, Buttery LD, Gross SS, Evans TJ, Seddon S, et al. Cytokine-stimulated expression of inducible nitric oxide synthase by mouse, rat, and human osteoblast-like cells and its functional role in osteoblast metabolic activity. Endocrinology. 1995. 136:5445–5453.

32. Marletta MA. Nitric oxide synthase: aspects concerning structure and catalysis. Cell. 1994. 78:927–930.

33. Govers R, Rabelink TJ. Cellular regulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol. 2001. 280:F193–F206.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download