Abstract
Purpose
Forkhead box protein 3 (FOXP3)+T cells are the major regulatory T cells controlling all aspects of the immune response. Transforming growth factor-β (TGF-β) is a suppressive cytokine which mediates the suppressive action of FOXP3+T cells. The aim of this study was to investigate the role of FOXP3+T cells, TGF-β in colonic mucosa of children with Crohn's disease (CD).
Methods
Colonic mucosal biopsies were obtained from 10 children with CD (12~15 years of age) and 11 control (8~15 years of age). Frequencies of FOXP3+T, CD4+T cells and TGF-β1 expression were examined in the lamina propria (LP) and lymphoid aggregates or follicles (LA/F) by immunohistochemistry, and later evaluated by association with disease activity.
Results
In the LP of CD group, frequencies of FOXP3+T, CD4+T cells, proportion of FOXP3/CD4+T cells and TGF-β1 expression significantly increased compared to the control. In the LA/F of CD group, frequency of FOXP3+T cells, proportion of FOXP3/CD4+T cells and TGF-β1 expression significantly increased compared to the control (p<0.05). CD4+T cells also increased compared to the control, but this finding was not significant. In the LP and LA/F of CD group, frequency of FOXP3+T cells exhibited positive correlation with CD4+T cells (p<0.05). In the LP and LA/F of CD group, TGF-β1 expression had positive correlation with CRP, Pediatric Crohn's Disease Activity Index, and negative correlation with hematocrit and albumin (p<0.05).
Figures and Tables
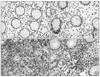 | Fig. 1Immunohistochemical staining of FOXP3 (×400). (A) In the lamina propria (LP) of Crohn's disease (CD) group. (B) In the LP of the control. (C) In the lymphoid aggregates or follicles (LA/F) of CD group. (D) In the LA/F of the control. |
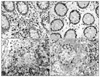 | Fig. 2Immunohistochemical staining of CD4 (×400). (A) In the LP of CD group. (B) In the LP of the control. (C) In the LA/F of CD group. (D) In the LA/F of the control. |
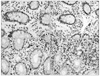 | Fig. 3Immunohistochemical staining of TGF-β1 (×400). (A) Grade 0 in the control, (B) Grade 1, (C) Grade 2, (D) Grade 3 in CD group. |
 | Fig. 4The frequencies of FOXP3+T, CD4+T cells, and the proportion of FOXP3+T cells to CD4+T cells and TGF-β1 expression were more increased in the lamina propria of Crohn's disease group (n=10) than the control (n=11). (A) FOXP3+T cells, (B) CD4+T cells, (C) FOXP3/CD4+T cells, (D) TGF-β1 expression. |
 | Fig. 5The frequencies of FOXP3+T, CD4+T cells, and the proportion of FOXP3+T cells to CD4+T cells and TGF-β1 expression were more increased in the lymphoid aggregates or follicles of Crohn's disease group (n=8) than the control (n=10). (A) FOXP3+T cells, (B) CD4+T cells, (C) FOXP3/CD4+T cells, (D) TGF-β1 expression. |
Table 1
Demographic and Clinical Characteristics of Patients with Crohn's Disease and Control Subjects

Table 2
Correlation of the Frequencies of FOXP3+T Cells with the Frequencies of CD4+T Cells, TGF-β1 Expression in CD Group

References
1. Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunology. Lancet. 2007. 369:1627–1640.
2. Kim JM. Inflammatory bowel diseases and enteric microbiota. Korean J Gastroenterol. 2010. 55:4–18.

4. Boden EK, Snapper SB. Regulatory T cells in inflammatory bowel disease. Curr Opin Gastroenterol. 2008. 24:733–741.

5. Allez M, Mayer L. Regulatory T cells: peace keepers in the gut. Inflamm Bowel Dis. 2004. 10:666–676.
6. Yu QT, Saruta M, Avanesyan A, Fleshner PR, Banham AH, Papadakis KA. Expression and functional characterization of FOXP3+ CD4+ regulatory T cells in ulcerative colitis. Inflamm Bowel Dis. 2007. 13:191–199.

7. Sitohy B, Hammarström S, Danielsson A, Hammarström ML. Basal lymphoid aggregates in ulcerative colitis colon: a site for regulatory T cell action. Clin Exp Immunol. 2008. 151:326–333.

8. Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, et al. Peripheral and intestinal regulatory CD4+ CD25 (high) T cells in inflammatory bowel disease. Gastroenterology. 2005. 128:1868–1878.

9. Saruta M, Yu QT, Fleshner PR, Mantel PY, Schmidt-Weber CB, Banham AH, et al. Characterization of FOXP3+CD4+ regulatory T cells in Crohn's disease. Clin Immunol. 2007. 125:281–290.

10. Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001. 27:20–21.

11. Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009. 30:636–645.

12. Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25+- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003. 198:1875–1886.

13. Fahlén L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, et al. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005. 201:737–746.
14. Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001. 194:629–644.

15. Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005. 201:1061–1067.

16. Shevach EM, Davidson TS, Huter EN, Dipaolo RA, Andersson J. Role of TGF-beta in the induction of Foxp3 expression and T regulatory cell function. J Clin Immunol. 2008. 28:640–646.

17. Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey H, O'Shea JJ, et al. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J Exp Med. 2008. 205:1975–1981.

18. Levéen P, Larsson J, Ehinger M, Cilio CM, Sundler M, Sjöstrand LJ, et al. Induced disruption of the transforming growth factor beta type II receptor gene in mice causes a lethal inflammatory disorder that is transplantable. Blood. 2002. 100:560–568.

19. La Scaleia R, Morrone S, Stoppacciaro A, Scarpino S, Antonelli M, Bianchi E, et al. Peripheral and intestinal CD4+ T cells with a regulatory phenotype in pediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2010. 51:563–572.

20. Bedossa P, Poynard T, Mathurin P, Lemaigre G, Chaput JC. Transforming growth factor beta 1: in situ expression in the liver of patients with chronic hepatitis C treated with alpha interferon. Gut. 1993. 34:S146–S147.

21. Hyams JS, Ferry GD, Mandel FS, Gryboski JD, Kibort PM, Kirschner BS, et al. Development and validation of a pediatric Crohn's disease activity index. J Pediatr Gastroenterol Nutr. 1991. 12:439–447.

22. Ricciardelli I, Lindley KJ, Londei M, Quaratino S. Anti tumour necrosis-alpha therapy increases the number of FOXP3 regulatory T cells in children affected by Crohn's disease. Immunology. 2008. 125:178–183.

23. Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008. 14:4280–4288.

24. Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006. 177:5852–5860.

25. Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med. 2006. 173:803–810.

26. Eastaff-Leung N, Mabarrack N, Barbour A, Cummins A, Barry S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol. 2010. 30:80–89.

27. Makita S, Kanai T, Oshima S, Uraushihara K, Totsuka T, Sawada T, et al. CD4+CD25 bright T cells in human intestinal lamina propria as regulatory cells. J Immunol. 2004. 173:3119–3130.

28. Holmén N, Lundgren A, Lundin S, Bergin AM, Rudin A, Sjövall H, et al. Functional CD4+CD25high regulatory T cells are enriched in the colonic mucosa of patients with active ulcerative colitis and increase with disease activity. Inflamm Bowel Dis. 2006. 12:447–456.

29. Babyatsky MW, Rossiter G, Podolsky DK. Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology. 1996. 110:975–984.

30. Ikeda M, Takeshima F, Ohba K, Ohnita K, Isomoto H, Yamakawa M, et al. Flow cytometric analysis of expression of transforming growth factor-beta and glucocorticoid-induced tumor necrosis factor receptor on CD4(+) CD25(+) T cells of patients with inflammatory bowel disease. Dig Dis Sci. 2006. 51:178–184.

31. Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J Clin Invest. 2001. 108:601–609.

32. Duchmann R, Zeitz M. T regulatory cell suppression of colitis: the role of TGF-beta. Gut. 2006. 55:604–606.

33. Vorobjova T, Uibo O, Heilman K, Rägo T, Honkanen J, Vaarala O, et al. Increased FOXP3 expression in small-bowel mucosa of children with coeliac disease and type I diabetes mellitus. Scand J Gastroenterol. 2009. 44:422–430.

34. Wang YF, Wei B, Ouyang Q. Expression with TGFbeta1 in the patients with ulcerative colitis. Sichuan Da Xue Xue Bao Yi Xue Ban. 2005. 36:204–206.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download