Abstract
Purpose:
Treatment of diabetic foot infection due to methicillin-resistant Staphylococcus aureus (MRSA) remains challenging. Applying vancomycin-impregnated cement is one of the best methods of treatment. Vancomycin-impregnated cement has been used worldwide; however, to date, there is a limited number of studies regarding its use. We evaluated the duration of antimicrobial activity of vancomycin-impregnated cement stored at room temperature after manufacturing.
Materials and Methods:
The vancomycin-impregnated cement was manufactured by mixing 1 g of vancomycin with 40 g of polymer and adding 17.90 g of liquid monomer. The cement dough was shaped into flat cylinders with diameter and height of 6 mm and 2 mm, respectively. Another cement of the same shape without mixing vancomycin was prepared as the negative control. All manufactured cements were sterilized with ethylene oxide gas and stored at room temperature. Each cement was placed on Mueller Hinton agar plate lawned with standard MRSA strain. Standard vancomycin disk and gentamicin disk were placed together. After 24 hours, the diameter of inhibition zone was measured, and if the diameter was less than 15 mm, vancomycin-impregnated cement was regarded as a loss of antimicrobial activity. The study was repeated every 2 weeks until vancomycin-impregnated cements lost their antimicrobial activity.
Results:
Vancomycin-impregnated cement stored for a duration of 16 weeks created a 14 mm inhibition zone, while vancomycin disk created a 15 mm inhibition zone. Vancomycin-impregnated cement stored for a duration of 17 weeks created 7 mm and 9 mm inhibition zones, while vancomycin disk created 16 mm and 15 mm inhibition zones, respectively.
Conclusion:
We found a decrease of antimicrobial activity in vancomycin-impregnated cements after 16 weeks. After 17 weeks, they showed definite loss of antimicrobial activity. Therefore, we recommend not using vancomycin-impregnated cement spacers that has been stored for more than 16 weeks at room temperature.
Go to : 
REFERENCES
1.Kim YJ., Chon S., Oh S., Woo JT., Kim SW., Rhee SY. Analysis of diabetes quality assessment findings and future directions for the appropriate management of diabetes in Korea. Korean J Intern Med. Published online March 15. 2017. DOI: doi: 10.3904/kjim.2016.136.
2.Shaw JE., Sicree RA., Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010. 87:4–14.

4.Berendt AR., Peters EJ., Bakker K., Embil JM., Eneroth M., Hinchliffe RJ, et al. Diabetic foot osteomyelitis: a progress report on diagnosis and a systematic review of treatment. Diabetes Metab Res Rev. 2008. 24(Suppl 1):S145-61.

5.Lipsky BA., Berendt AR., Cornia PB., Pile JC., Peters EJ., Armstrong DG, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012. 54:e132–73.
6.Wininger DA., Fass RJ. Antibiotic-impregnated cement and beads for orthopedic infections. Antimicrob Agents Chemother. 1996. 40:2675–9.

7.Roeder B., Van Gils CC., Maling S. Antibiotic beads in the treatment of diabetic pedal osteomyelitis. J Foot Ankle Surg. 2000. 39:124–30.

8.Nelson CL., McLaren SG., Skinner RA., Smeltzer MS., Thomas JR., Olsen KM. The treatment of experimental osteomyelitis by surgical debridement and the implantation of calcium sulfate tobramycin pellets. J Orthop Res. 2002. 20:643–7. 9. LoGerfo FW, Coffman JD. Current concepts. Vascular and microvascular disease of the foot in diabetes. Implications for foot care. N Engl J Med. 1984;311: 1615-9.

10.Cunningham A., Demarest G., Rosen P., DeCoster TA. Antibiotic bead production. Iowa Orthop J. 2000. 20:31–5.
11.Traub WH., Leonhard B. Heat stability of the antimicrobial activity of sixty-two antibacterial agents. J Antimicrob Chemother. 1995. 35:149–54.

12.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: twenty-third informational supplement. Wayne, PA: Clinical and Laboratory Standards Institute;2013.
13.Nelson CL., Hickmon SG., Harrison BH. Elution characteristics of gentamicin-PMMA beads after implantation in humans. Ortho- pedics. 1994. 17:415–6.

14.Salvati EA., Callaghan JJ., Brause BD., Klein RF., Small RD. Reimplantation in infection. Elution of gentamicin from cement and beads. Clin Orthop Relat Res. 1986. 207:83–93.
15.Scharer BM., Sanicola SM. The in vitro elution characteristics of vancomycin from calcium phosphate-calcium sulfate beads. J Foot Ankle Surg. 2009. 48:540–2.

16.Balsamo LH., Whiddon DR., Simpson RB. Does antibiotic elution from PMMA beads deteriorate after 1-year shelf storage? Clin Orthop Relat Res. 2007. 462:195–9.

Go to : 
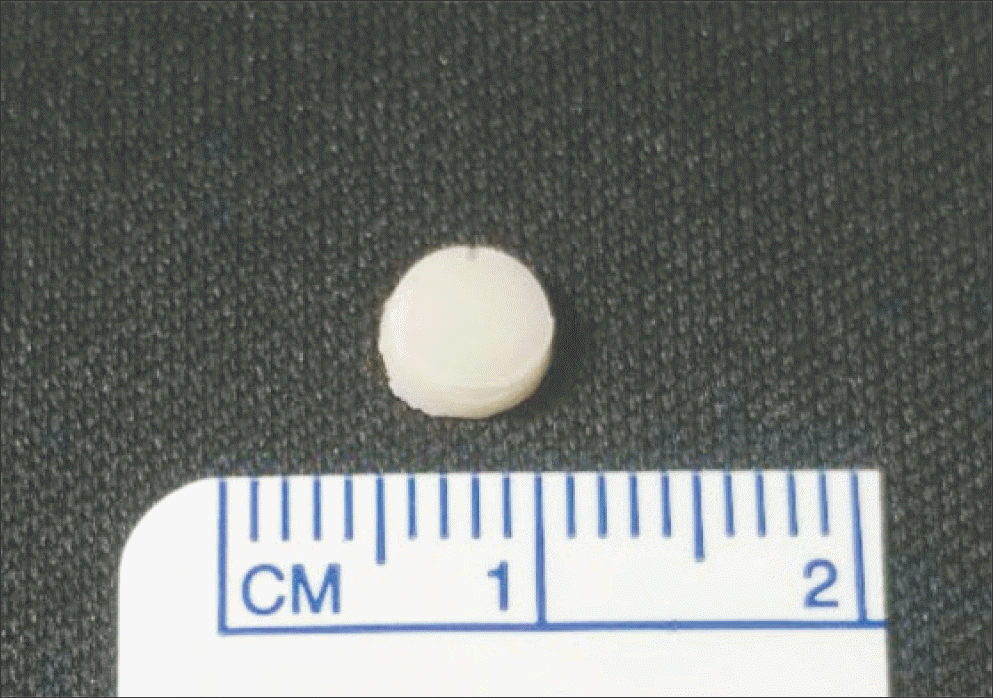 | Figure 1.The antibiotics-impregnated cements were manufactured as a disk form 6 mm diameter and 2 mm height. |
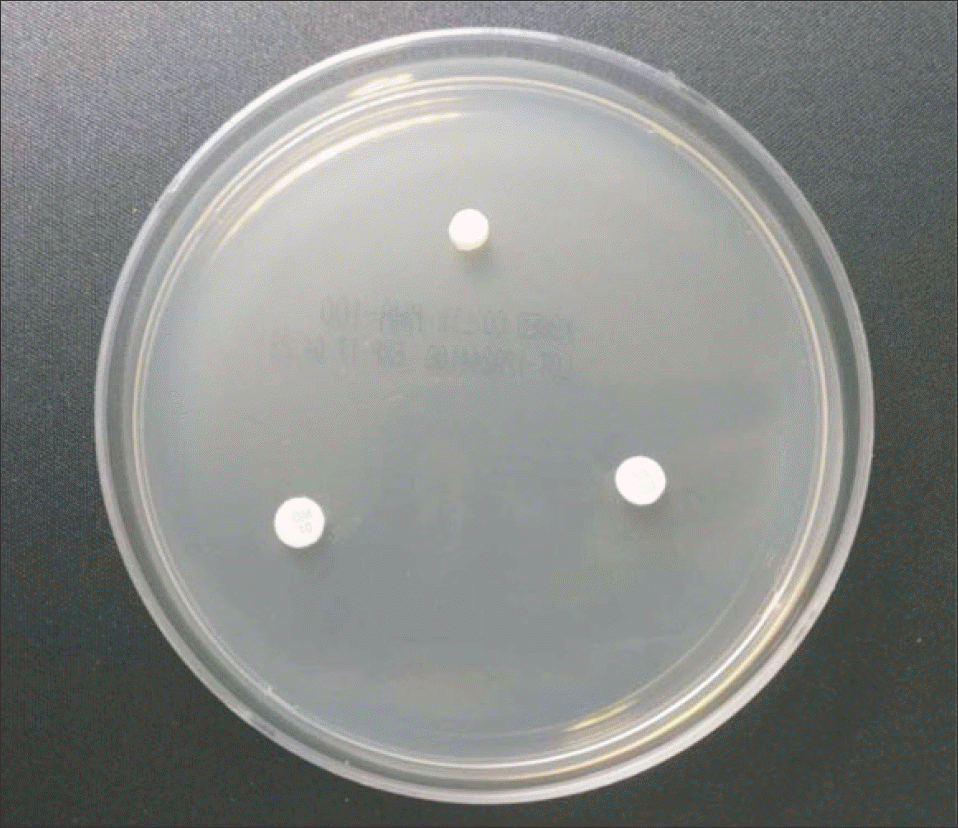 | Figure 2.The vancomycin-impregnated cement and the gentamicin-impregnated cement were placed on Mueller Hinton agar plate lawned with methicillin-resistant Staphylococcus aureus. Commercially available vancomycin disk and gentamicin disk were placed on the same plate. Each cement and disk was placed with at least 24 mm distance. |
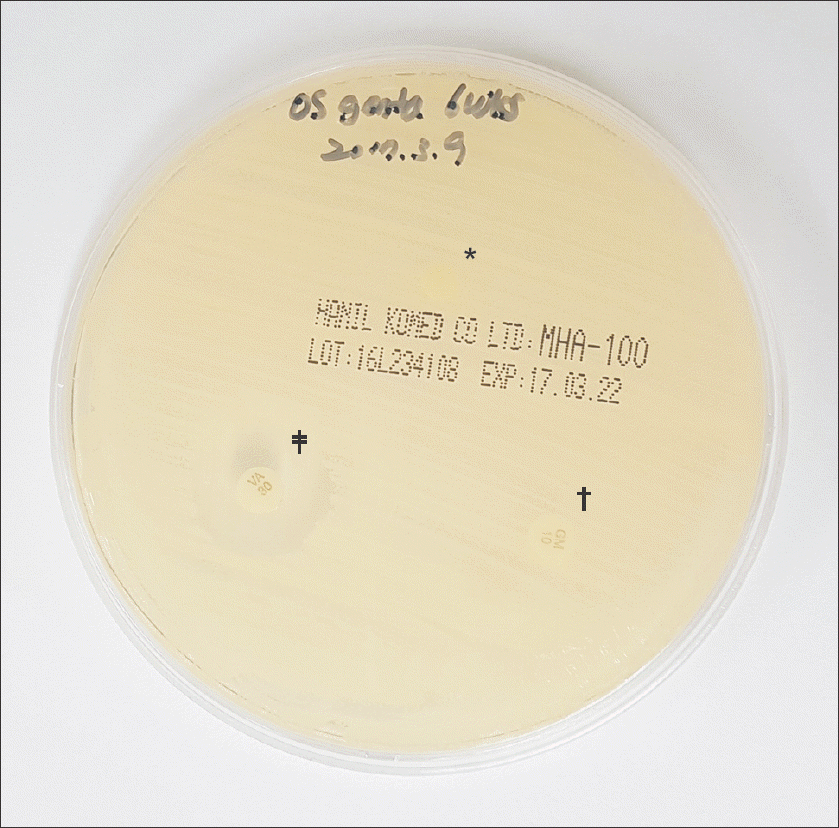 | Figure 3.In every agar plate, the gentamicin-impregnated cements (*) and gentamicin disks (†) as negative control didn’t create inhibition zones while vancomycin disks (‡) as positive control created inhibition zones after 24 hours incubation which were larger than 15 mm wide. |
 | Figure 4.The vancomycin-impregnated cement, vancomycin disk and gentamicin disk were placed on the Mueller Hinton agar plate lawned with methicillin-resistant Staphylococcus aureus (A) and incubated for 24 hours. The vancomycin-impregnated cement stored for 17 weeks (B) created 7 mm inhibition zone while vancomycin disk as positive control (C) created 16 mm inhibition zone after 24 hours incubation. |
 | Figure 5.The vancomycin-impregnated cement, vancomycin disk and gentamicin disk were placed on the Mueller Hinton agar plate lawned with methicillin-resistant Staphylococcus aureus (A) and incubated for 24 hours. The vancomycin-impregnated cement stored for 17 weeks (B) created 9 mm inhibition zone while vancomycin disk as positive control (C) created 15 mm inhibition zone after 24 hours incubation. |
Table 1.
The Inhibition Zone Diameter Depending on the Storage Periods




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download