Abstract
Modern medical technology aligns with medical device advancement, and new medical devices create the need for new procedure techniques. Unmet needs that physicians experience in clinical practice suggest solutions to problems; to solve these, collaboration between medical device manufacturers and physicians becomes a starting point for new medical device development. Commercialization of medical devices is greatly impacted by the product regulatory approval system, health technology assessment, and reimbursement system. Speed to market plays a far greater role in the medical device market than in the pharmaceutical market. Considering the current trend of evidence-based medicine and value-based pricing, efforts to generate clinical evidence should be strengthened even further while a greater focus should be placed on efforts to introduce global multicenter pre-market clinical trials in the Republic of Korea through strong collaboration with global companies. Since the strength and quality of clinical evidence is comparatively low in medical device studies, this could affect the decision making process and raise the issue of uncertainty. To overcome this issue, a risk-sharing system in the medical device field and 'coverage with evidence development' should be introduced; by doing so, evidence generation opportunities can be created without burying innovative technology and solving the issue of uncertain decisions. In addition, reimbursement coverage to support the costs of clinical studies needs to be established for early evidence generation, as seen in other countries.
현대 의료기술은 의료기기의 발전과 궤를 같이 하고 있으며 새로운 의료기기 개발이 신의료기술을 창출한다는 것이 새삼스럽지 않은 현실이다. 새로운 의료기기가 새로운 의료행위(술기)를 창출한다. 새로운 의료기기 개발은 주로 의료기기 제조회사와 의사간 협력으로 이루어지며 이로 인한 제품 상용화의 성공률 또한 매우 높아지게 된다. 의사들이 임상현장에서 경험하는 '미충족 니즈(unmet needs)'는 문제해결의 단초를 제공하며 이를 해결하기 위한 제조회사와 의사 상호간 협력이 신의료기기 개발의 출발점이 된다. 미국의 경우, 1990-1996년에 출원된 약 26,000건의 의료기기 특허 가운데 약 20%가 의사가 출원한 것이며 비의사가 출원한 특허보다 의사가 출원한 특허가 의료기기 개발에 더 많은 영향력을 끼쳤음을 알 수 있다[1].
전세계적으로 의료기기 산업을 국가발전 신성장 동력으로 선정하고 이를 육성 발전시키고자 하는 청사진을 제시하는 국가들을 어렵지 않게 찾아볼 수 있다. 전세계 의료기기 시장의 약 39%를 차지하고 있으며 실제적으로 의료기기 혁신개발을 독점하다시피 하고 있는 미국은 접어두고, 우리나라 주변의 일본과 중국을 포함한 대다수 서구 선진국가들에서 의료기기 산업을 촉진시키고자 노력하고 있다. 우리나라도 예외는 아니어서 과거 정부부터 현정부에 이르기까지 의료기기산업 발전계획을 끊임없이 발표해 왔다. 최근에는 '2020년, 세계 7대 의료강국 진입을 위한 '의료기기산업 중장기 발전계획'을 발표한 바 있다[2]. 의료기기산업에 대한 높은 관심과 정부차원의 목표달성을 위한 전략수립 및 실행은 고무적이나, 현실성이 결여된 과도한 목표설정과 이로 인한 잘못된 전략은 목표달성을 이루지 못할 경우에 발생하는 상실감과 부적절한 투자로 인한 자원낭비 등과 같은 자칫 적지 않은 부작용을 유발할 수 있다. 무엇보다도 의료기기산업에 대한 명확한 이해가 의료기기 강국을 위한 첫걸음이 될 것이다.
의료기기법 제2조(정의)에 따르면, '의료기기'란 사람이나 동물에게 단독 또는 조합하여 사용되는 기구·기계·장치·재료 또는 이와 유사한 제품으로서 질병을 진단·치료·경감·처치 또는 예방할 목적으로 사용되는 제품, 상해 또는 장애를 진단·치료·경감 또는 보정할 목적으로 사용되는 제품, 구조 또는 기능을 검사·대체 또는 변형할 목적으로 사용되는 제품, 임신을 조절할 목적으로 사용되는 제품 등으로 구분된다. 이와 같은 의료기기 정의는 나라마다 일부 차이는 있겠으나 전체적으로 대동소이한 모습을 띠고 있다. 의료기기의 종류와 범위는 안경 및 콘택트렌즈와 같은 일반 소비성 제품, 혈압기, 체온기, 혈당측정기와 같은 비교적 간단한 홈케어 의료기기, 인공심장 박동기, 인공관절, 인조혈관, 자기공명영상, 컴퓨터단층촬영과 같은 최첨단 의료기기(장비)에 이르기까지 매우 다양하다. 편의상 의료기기의 다양성을 구분하면 Table 1과 같다.
전세계 의료기기 시장 규모는 약 3,090억 달러(2012년), 국내시장 규모는 49억 달러(2012년)로 세계 11위(전세계 의료기기 시장의 1.6%)로 추정된다. 산업적 특성을 살펴볼 때 의료기기산업은 통상 첨단기술 및 지식이 집약된 고부가가치 산업이며 기술 및 규제장벽이 높다. 그러나, 위와 같은 의료기기 정의에서 미루어 짐작하듯이 모든 의료기기가 반드시 첨단기술의 고부가가치 제품만으로 구성되어 있는 것은 아니라는 점을 살펴볼 필요가 있다. 새로운 진입자가 의료기기 산업에 뛰어들 때 어떠한 제품을 가지고 시장에 진입하느냐에 따라 상이한 진입경로를 취할 수 있다. 일반적으로 의료기기 산업은 제약산업과 차별되는데, 짧은 연구개발 및 상용화 기간과 소규모의 연구개발 투자비용의 특성 때문에 의료기기 개발 후발주자인 우리나라가 국제적인 경쟁력을 갖추기에 유리하다고 볼 수 있으며 중소기업형 산업에 보다 적합한 업종이다. 이와 같은 상황은 외국뿐만 아니라 우리나라에서도 공히 적용되어 의료기기 제조회사의 수가 매우 많고 회사별 매출과 종업원수는 적은 중소기업의 특성을 엿볼 수 있다. 국내 제조업체의 경우 2013년 기준, 종업원 20인 미만 업체는 2,143개 소(83.8%)이며 100인 이상 종업원 업체는 60개 소(2.3%)에 불과하다(Table 2) [3]. 제약산업과 차별화되는 의료기기산업의 특징은 Table 3와 같다[4].
제품 인허가제도, 신의료기술평가제도, 보험급여제도는 의료기기 상용화에 지대한 영향을 끼친다. 제약산업에서도 제품 상용화를 위한 조속한 시장 진입이 중요하겠으나, 의료기기의 경우 시장진입 속도의 중요성은 제약산업보다 훨씬 더 크다. 이는 의료기기산업의 특성(짧은 제품주기 등)에 기인하는 바가 크다. 제품 상용화를 위한 규제장벽을 계획된 기간 내에 넘어서지 못할 경우 기존의 경쟁회사뿐만 아니라 새로운 경쟁 진입자의 출현으로 인해 시장 주도권을 빼앗기며 이는 결국 제품 상용화 실패로 이어진다. 따라서 기업의 성패에 있어서 제품 연구개발 능력 못지 않게 규제 장벽을 효율적으로 뛰어 넘어설 수 있는 시장진입을 위한 규제과학(regulatory science) 분야의 역량이 매우 중요하며 그 중요성은 더욱 더 커지고 있다. 결국 시장진입 속도가 사업 성패를 좌우하게 된다.
의료기기 개발은 제품 전 주기(total product life cycle) 모형을 따르는데, 아이디어 창출, 전 임상, 임상, 제조, 차세대 제품 개념 개발 및 디자인으로 이어지는 순환적이고 반복적인 과정을 거친다. 현행 상용화된 제품의 임상경험을 바탕으로 지속적으로 새로운 제품이 개발되며 이러한 과정을 통해 제품의 제조 비용이 절감되고 품질 개선이 점진적으로 이루어진다(Figure 1) [5]. 제품 전 주기는 미국 식품의약국(US Food and Drug Administration)에서도 의료기기 특성을 반영하는데 이용하는 모형이다. 또한, 의료기기 개발 과정은 환류고리와 제품개선의 연속체이다(Figure 2) [5]. Figure 2에서는 명확하게 구분되는 단계(가령, 전 임상 혹은 임상)로 묘사되어 있지만, 제품시험과 사용경험이 축적됨에 따라 의료기기 개발 단계는 중첩되거나 반복되며 이러한 과정을 거쳐 시판 가능한 제품개발에 더욱 다가서게 된다. 시장진입 후, 시장에서의 제품평가와 이를 통한 제품 개선이 지속적으로 이루어진다.
여타 산업과 마찬가지로 시장진입에 있어 선택과 집중 전략은 매우 중요하다. 의료기기 산업의 경우 폭 넓은 다양성과 복잡성으로 인해, 진입하고자 하는 의료기기 세부시장 선정과 제품 포지셔닝이 매우 중요하다. 제약산업과 비교할 때 비교적 적은 투자비용으로 시장진입이 가능하다는 점은 분명 매력적이나, 전세계 의료기기시장에서 소수의 글로벌 기업이 이미 대다수 시장을 점유하고 있고, 고부가가치 품목 분야에서는 이들 기업의 기술장벽이 매우 높다. 국내에서는 드물게 발생하지만 서구 선진국에서는 특허소송이 매우 빈번히 발생하고 있어 실제적으로 해외시장 진입이 쉽지만은 않다. 국내의 협소한 의료기기시장 규모를 살펴볼 때, 궁극적으로 수출을 염두 해 두지 않고는 기업의 규모를 키워 글로벌기업으로 성장하기에는 한계가 있다. 따라서, 진입하고자 하는 의료기기 세부 시장 선정과 제품개발, 특히 의료기기의 특성상 짧은 제품주기로 인한 지속적인 신제품 개발을 이끌어낼 수 있는 끊임없는 연구개발 투자를 확보해야 한다.
2012년도 국가별 의료기기 기업의 매출액 대비 연구개발비는 중국(9.8%), 미국(7.6%), 유럽(5.9%), 한국(5.7%)로 보고되고 있다. 우리나라의 경우 연구개발비는 매출 대비 4.2%(2009)에서 5.7%(2012)로 연평균 17.2%의 높은 증가율을 보여 매우 고무적이다[6]. 외견상 우리나라 연구개발 비율이 유럽과 유사해 보이나 찬찬히 살펴보아야 한다. 유럽의 경우 국내기업과 비교 시 매출규모도 매우 클 뿐만 아니라 소수의 토종 유럽계 글로벌기업(지멘스, 필립스 등)을 제외하고는 미국계 글로벌기업이 유럽시장 또한 장악하고 있다. 거의 대다수 미국계 글로벌기업은 유럽에 현지 공장 및 연구소를 운영하고 있을 뿐만 아니라, 과거 10년간 제약산업과 마찬가지로 의료기기산업에서도 대규모 인수합병이 이루어져 왔고 이 와중에 유럽계 글로벌기업이 미국회사에 흡수 합병된 사례가 적지 않다. 주요 글로벌기업의 경우 매출액 대비 연구개발비가 10%를 상회하며 우리나라와는 여러모로 격차가 크다. 국내기업이 진출하고자 하는 의료기기 세부시장에 맞는 연구개발 투자가 지속적으로 이루어져야만 지속적인 생존 및 성장이 가능하다[7].
최근 전세계 의료기기시장은 다양한 변화에 직면하고 있다. 가격인하 압력, 과다 경쟁, 신흥국 제조자(중국)의 약진, 모바일 헬스(mobile health)로 대표되는 새로운 시장 진입자 출현, 환자의 권리 신장, 인허가 규제 및 시판 후 품질관리 강화, 가치중심 보험급여, 그리고 혁신기술의 고갈 등이 나타나고 있다. 이러한 변화는 2008년 9월 발생한 미국 투자은행 리먼브라더스 파산으로 촉발된 글로벌 경제위기 후 점진적으로 나타나다가 최근 2-3년 동안 변화의 폭과 속도가 더욱 커져가고 있다. 최근의 의료기기 시장 추세는 가치 중심 헬스케어로의 전환, 안전성 및 유효성 입증 강화를 위한 규제 압력, 그리고 자원의 제약으로 요약되며, 의료기기산업을 뒤흔드는 최악의 상황이라고 회자되고 있다. 이 가운데 가치 중심 헬스케어로의 전환에 대한 배경으로 지목된 요인—지속적인 의료비 상승, 보건의료 재정 압박, 그리고 미충족 니즈—에 대해 이를 해결하기 위한 방편으로 비교효과연구, 의료기술평가, Accountable Care Organization 그리고 질병관리프로그램 등이 활발히 진행되고 있다[8].
위에 열거된 의료기기 시장추세와 의료기술 개발 및 국제화 측면을 살펴볼 때, 결국에는 의료기기 시장진입에 필수 불가결하게 요구되는 임상 가치에 대한 근거, 그리고 이와 더불어 점진적으로 요구되고 있는 경제적 가치를 입증할 수 있는 근거마련이 핵심이라고 할 수 있다. 과거에는 안전성 및 유효성 입증의 틀에 국한되었던 요구사항이 효과성뿐만 아니라 보험자 관점에서의 투자 효율성과 가치에 대한 측면으로 확장되고 있다. 우수한 기술이 개발되어도 이를 뒷받침할 수 있는 근거창출 노력을 소홀히 할 경우 연구개발 투자에 상응하는 적절한 가격산정은 고사하고 시장진입조차 어려워지는 상태에 직면하게 된다. 이와 같은 상황은 전세계적인 추세이며 우리나라의 경우 2007년 신의료기술평가제도 도입 후 의료기기업계에 더욱 절실하게 다가오고 있다.
의료기기기업은 새로운 의료기술개발의 주체로서 인허가, 신의료기술평가, 보험 급여 등과 같은 규제장벽을 극복하기 위한 노력에 매진해 왔다. 최근의 의료기기 시장 환경 변화에 적극 대처하기 위해서는 무엇보다 임상 및 경제성 근거 창출에 보다 집중해야 한다. 경제적 가치의 근거 창출은 양질의 임상적 근거가 창출되어야만 비로소 후속적으로 진행될 수 있기에 의료기기 특성상 현재 대다수 의료기기의 임상적 근거가 빈약한 상황에서 경제적 가치 창출 가능성은 제한적이라 볼 수 있다. 그러나, 근거중심의학과 가치중심 가격산정이라는 전세계적인 동향을 고려할 때 임상적 근거 창출 노력은 현재보다 더욱 더 강화할 필요가 있다.
일반적으로 우리나라 식품의약품안전처를 비롯한 전세계 의료기기 인허가기관에서는 안전성 및 유효성 그리고 성능입증을 요구하고 있으나 실제 의료기기의 가치를 입증하기에는 부족한 부분이 있다. 인허가 당시에는 무작위비교임상연구는 거의 찾아보기 어려우며 설령 있다 하더라도 우위 임상연구보다는 비열등 임상연구가 주를 이룬다. 이와 같은 배경에는 인허가의 경우 기존 제품과의 실체적 동등성 여부가 인허가 판단의 기준이 되기 때문이다. 또한, 실제 우위 임상연구를 실시하기 위해 요구되는 대규모 환자수를 감안할 때 수반되는 임상연구 비용은 매우 큰 부담이다. 근거 창출에는 많은 비용이 수반되며 우수한 제품이라 할지라도 임상근거가 미흡하면 그 가치를 인정받지 못한다. 실제로 유럽에서 승인된 많은 의료기기(특히, 임상연구가 요구되는 고위험 3등급 의료기기)가 미국시장에는 진입하지 못하는 경우가 많은데 미국식품의약국 승인을 위해 요구되는 임상비용을 충당하기 어렵기 때문이다. 또한, 미국식품의약국에서 요구되는 엄격한 임상연구 요구조건으로 인해 유럽에서의 판매허가 시점과 미국의 판매허가 시점간의 격차는 과거에 비해 점점 더 벌어지고 있다.
또한, 판매허가를 위해 실시한 임상연구 결과를 실세계의 임상현장에 모두 적용하기에는 한계가 있어 통상 판매허가 후 임상연구를 실시하게 되며 유효성보다는 효과성에 더 무게가 실리게 된다. 바로 이러한 점이 국내 신의료기술평가제도 운영상에 관련 이해당사자간 갈등의 원인이 된다. 의료기기업계는 식품의약품안전처의 허가를 받은 의료기기가 시장진입을 못하는 현실에 불만을 갖고 있는 반면(외국의 경우 인허가기관에서 승인된 제품은 의료현장에서 바로 사용이 가능하다), 신의료기술평가제도를 관할하는 보건복지부나 한국보건의료연구원 입장에서는 다른 국가들과는 구분되는 우리나라의 보건의료제도 상황(특히, 지속적으로 비급여 시장이 확대되고 있는 상황)에서 초기에 의료기술의 안전성 및 유효성, 효과성까지 검증하고자 하는 정책적 동인이 있어 이해당사자간 구조적 갈등관계에 놓여 있다. 최근 신의료기술평가를 보다 유연하게 운영 하고자 하는 정부의 노력으로 과거보다는 향후 의료기기업계 및 의료수요자의 불만은 잦아들 것으로 예상되나, 궁극적으로 임상적 근거 확충에 대한 근본적인 문제는 해결해야 할 과제이다.
궁극적으로 의료기기 제조사는 임상근거 창출을 위한 노력을 더욱 기울여야 하며, 의료기관은 임상연구 수준을 지속적으로 높여야 하며, 정부는 임상근거 창출에 요구되는 임상연구 인프라 구축 및 지원을 마련할 필요가 있다. 높은 수준의 임상연구 능력은 의료기술 개발의 토대이다. 국내에서 활성화된 허가 후 임상의 지속적인 수행뿐만 아니라 허가 전 임상연구 수행능력을 강화해야 한다. 이를 위해서 글로벌 기업과의 협력을 통해 국제 다기관 허가 전 임상연구를 국내로 유치할 필요가 있다. 현재 국제 다기관 허가 전 임상연구의 국내 유치에 가장 큰 걸림돌로 지적되고 있는 요인으로 임상연구 비용부담을 들 수 있다[9]. 선진 외국의 경우, 의료기기 상용화 촉진과 신기술에 대한 환자 혜택 강화 측면에서 임상연구의 중요성을 인식하여 의료기기 임상연구 비용을 보험급여로 지원하고 있다. 미국의 경우, 2000년부터 임상시험에 메디케어(Medicare)를 적용하고 있는데, 치료목적 임상시험, 근거중심 치료법 개발 목적의 임상시험과 일반적 치료비용(표준치료 및 검사, 진료비 등) 등을 보험급여로 인정하고 있다. 또한, 공보험인 메디케어뿐만 아니라 사보험에서도 임상연구에 대해서 메디케어와 동일하게 급여로 인정하고 있다. 서구 유럽도 미국과 유사한 형태의 보험급여를 제공하고 있으며, 일본과 호주의 경우에서도 임상연구에 대하여 일정 형태의 보험급여 혜택을 제공하고 있다. 의료기기 임상연구와 관련하여 임상연구 인력 연구비, 환자 보험 비용, 데이터 모니터링 비용 등에 대해서는 임상연구 지원자(제조사)가 부담하게 되지만, 의료기기 임상연구와 관련하여 수반되는 입원비, 검사비, 약제비, 재료대, 수술비 등에 대해서는 보험자가 부담하고 있다(Table 4) [101112]. 제약과 달리 의료기기는 임상시험에 수반되는 환자당 임상연구 비용부담이 더욱 높다. 임상연구가 요구되는 대다수 의료기기는 수술실에서 사용되기 때문에 환자의 입원치료가 필수적이며 입원료, 검사료, 수술료, 중환자실 비용, 약제비 등의 환자 재원기간 동안 소요되는 매우 포괄적인 비용이 발생한다. 임상연구의 건강보험 지원이 재정상의 문제로 어려울 경우 '연구중심병원'에라도 제한적으로 보험급여로 임상연구 비용을 지원할 경우 임상연구 활성화와 더불어 임상적 근거 창출에 기여할 것으로 기대된다.
또한, 임상연구 비용지원과 더불어 비용 효율적인 방법으로 임상연구를 촉진하는 방법을 모색할 필요가 있다. 최근 국제저널에 발표된 비용 효율적인 임상연구 실행이 주목을 받고 있는데 전자의무기록과 개선된 정보처리 통합기술을 이용한 레지스트리에 기초한 무작위 임상연구는 연구기관과 비용을 현저히 줄이면서(많게는 99%까지) 양질의 임상연구 결과를 도출하였다. 일부에서는 이러한 연구를 임상연구 분야의 파괴적 기술이라고 일컫기도 한다. 전통적인 임상연구의 경우 비용이 5천만 달러 이상 소요되는 경우도 빈번하나, 스웨덴에서 7,244명 환자를 대상으로 실시된 전향적, 다기관, 무작위 비교 임상연구인 TASTE연구는 ST분절 상승형 심근경색(ST-segment elevation myocardial infarction) 환자의 혈전 흡입 효과를 살펴보기 위한 임상연구로서 환자당 50달러 정도로 전체 임상연구 비용이 50만 달러 미만이 소요되었다[13]. 전통적인 무작위 임상연구가 여전히 연구의 표준이지만 비용과 시간소모가 많고 이로 인해 자원투입에 많은 어려움을 겪고 있는 것이 현실적 문제이다. 특히, 시장성이 높지 않은 의료기술에 대해서는 대규모 무작위 임상시험이 이루어지기 어려운 상황을 고려할 때, 레지스트리에 기초한 연구는 데이터 완성도, 데이터 타당성, 모니터링, 임상연구 디자인 등에서 해결할 과제가 있음에도 불구하고 근거가 불충분한 의료기술 연구분야에 대규모 환자를 대상으로 근거 간극을 보완할 수 있는 연구방법으로 판단된다.
의료기기는 다양성이 매우 높고 점진적인 기술 혁신이라는 특성을 갖는 고부가가치 산업이다. 전세계 의료기기 시장은 다수의 중소기업과 소수의 대기업으로 이루어져 있으며 대기업과 더불어 다수의 연구개발 중심의 스타트업(start-up) 기업들이 의료기기 혁신 개발에 많은 기여를 하고 있으며 대기업과의 협력 혹은 추후 인수합병을 거쳐 혁신기술이 상용화 길을 걷게 된다. 2008년 전세계 경제위기 발생 이후 의료기기 산업 또한 중대한 변환기를 맞이하고 있으며, 각국의 보건의료 재정에 대한 압박이 커져가고 있는 상황에서 비용에 대한 가치 입증 요구가 증가하고 있다. 과거의 혁신기술 제품개발과 인허가 승인이 제품의 환자 접근성을 충족시켰던 시기는 이미 지났으며 제품의 가치 입증을 위한 근거 창출 노력이 제조회사에 더욱 더 요구되고 있다. 제품 연구개발 초기단계부터 임상(더 나아가 경제성) 근거 창출을 위한 전략 수립과 실행이 요구된다. 제약산업과 달리 의료기기산업의 경우 시장진입 속도 촉진 중요성은 더욱 높으며 이는 짧은 제품 생명주기에 기인한다. 특허를 통해 독점성이 보장되는 제약산업과 달리, 의료기기산업은 후발기업이 혁신기술 개발 선발기업보다 그 수혜를 더 누릴 수 있는 가능성이 매우 높다. 따라서, 선발기업은 빠른 시일 내에 인허가, 신의료기술평가, 보험급여라는 모든 규제장벽을 넘어서야만 혁신에 대한 대가를 누릴 수 있게 된다. 이는 빠른 추격자 전략이 보다 많은 혜택을 받게 되는 잘못된 동기부여를 제공하여 지속적인 혁신기술 개발을 저해하는 요인이 된다. 기업은 규제장벽을 빨리 뛰어넘을 수 있는 규제과학 분야의 역량을 강화하는 한편, 규제 당국은 혁신제품의 시장진입 관련 의사결정 속도를 촉진시킬 수 있는 제도 마련을 통해 지속적으로 의료기기 혁신이 이루어질 있도록 해야 한다. 현재 식품의약품안전처와 한국보건의료연구원의 인허가와 신의료기술평가를 동시에 진행하는 동시검토제도(원스톱 서비스)는 좋은 사례이다. 보험급여 결정 과정에서도 행위와 치료재료의 순차적 급여결정 절차가 아니라 동시에 이루어질 수 있는 제도 운영이 요구된다.
의료기기 특성상 임상근거의 강도와 질이 낮아 의사결정 시 불확실성 문제가 초래되고 있다. 이러한 문제에 대한 해결책으로 국내 제약분야 및 외국 의료기기 분야에서 적용하고 '위험분담제도(risk sharing)'를 국내 의료기기 분야에 도입하고 '근거창출을 위한 조건부 급여(coverage with evidence development)' 제도를 시행하여 혁신기술을 사장시키지 않고 근거창출 기회를 마련하여 의사결정의 불확실성 문제를 해결하는 방안이 요구된다. 또한, 보다 조기에 근거창출을 위한 지원 방법으로 외국의 사례에서와 같이 임상연구에 소요되는 비용의 보험급여 지원제도를 마련할 필요가 있다.
본 논문은 보건의료 기술 발전과 함께 새로운 의료기기, 이로 생성되는 의료행위의 시장진입, 허가, 사용 등에 대해 산업체의 시각에서 향후 방향을 제안한 논문이다. 의료기술이란 제약산업과는 다른 특성을 지니고 있는데, 본 논문에 그 차이를 구체적으로 잘 비교하였으며 의료기술의 확산에 필요한 기업의 역할에 대해 구체적이고 현실적인 내용을 중심으로 기술하였으며, 특히 주요국 의료기기 관련 정책과 의료기기의 특성 및 의약품과의 차이점을 자세히 기술하였다. 외국과의 비교는 물론 국내의 의료특성을 감안한 비교평가가 이루어져 있으며 정책적으로 기업이 할 일은 물론 국내 의료기술의 발전 및 세계화를 위한 방향성 제시도 일부 되어있다는 점에서 의의가 있는 논문이라 판단된다.
[정리: 편집위원회]
Figures and Tables
Figure 1
Total product life cycle (From US Food and Drug Administration. Medical device innovation initiative white paper: CDRH Innovation Initiative [Internet]. Silver Spring: US Food and Drug Administration; 2011) [5].
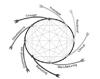
Figure 2
Medical device development pathway from discovery and ideation to product launch and post market monitoring (From US Food and Drug Administration. Medical device innovation initiative white paper: CDRH Innovation Initiative [Internet]. Silver Spring: US Food and Drug Administration; 2011)[5].

References
1. Chatterji AK, Fabrizio KR, Mitchell W, Schulman KA. Physi-cian-industry cooperation in the medical device industry. Health Aff (Millwood). 2008; 27:1532–1543.

2. Ministry of Health and Welfare. Mid-/long-term development plan for medical device industry aiming at global 7th rank by 2020 [Internet]. Sejong: Ministry of Health and Welfare;2014. cited 2014 Nov 3. Available from: http://www.mw.go.kr/front_new/al/sal0301vw.jsp?PAR_MENU_ID=04&MENU_ID=0403&CONT_SEQ=299308&page=1.
3. Ministry of Food and Drug Safety. Report on production, export and import of medical device in 2013. Cheongju: Mini-stry of Food and Drug Safety;2013.
4. Korea Medical Devices Industry Association. Position paper. Seoul: Korea Medical Devices Industry Association;2013.
5. US Food and Drug Administration. Medical device inno-vation initiative white paper: CDRH Innovation Initiative [Internet]. Silver Spring: US Food and Drug Administration;2011. cited 2014 Nov 3. Available from: http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDRH/CDRHInnovation/ucm242067.htm.
6. Kim SB. Business performance analysis of medical device companies by countries in 2012. KHIDI Brief. 2013; 75:[Epub]. http://info.khidi.or.kr/www/download.jsp?i=5429.
7. Seo GS. Analysis on market size by medical device sectors and R&D investment status. KHIDI Brief. 2012; 20:[Epub]. http://medicaldevice.khidi.or.kr/board.es?mid=a10401000000&bid=0004&act=view&list_no=457.
8. Ernst & Young Global Limited. Pulse of the industry: medical technology report 2013 [Internet]. London: Ernst & Young Global Limited;2013. cited 2014 Nov 3. Available from: http://www.ey.com/US/en/Industries/Life-Sciences/Pulse-of-the-industry---medical-technology-report-2013.
9. Korea Medical Devices Industry Association. The proposal to Ministry of Health and Welfare about medical device clinical research policy. Seoul: Korea Medical Devices Industry Association;2012.
10. Medicare Program; Criteria and Procedures for Extending Coverage to Certain Devices and Related Services, 42 C.F.R. 405. 1995.
11. Public Health Code Article, L 1121-16-1. 2011.
12. Hospital Remuneration Act, KHEntgG, No. 8. 2002.
13. Frobert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gud-nason T, Maeng M, Aasa M, Angeras O, Calais F, Danielewicz M, Erlinge D, Hellsten L, Jensen U, Johansson AC, Karegren A, Nilsson J, Robertson L, Sandhall L, Sjogren I, Ostlund O, Harnek J, James SK. TASTE Trial. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013; 369:1587–1597.





 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download