Abstract
Figures and Tables
 | Figure 1Overview of the Mini-Sentinel safety question evaluation process. a)Only those academic institutions with electronic healthcare data will receive safety questions for evaluation. b)Data partners will provide summary results from analysis conducted within their secure data environments. Those summary results will not include directly indentifiable health information. From US Department of Health and Human Services, US Food and Drug Administration. The Sentinel Initiative: a national strategy for monitoring medical product safety [Internat]. Silver Spring: US Food and Drug Administration; 2011 [13]. |
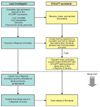 | Figure 2European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) study process flow chart. CoC, code of conduct; DoI, declaration of interests (From European Network of Centres for Pharmacoepidemiology and Pharmacovigilance. ENCePP studies [Internet]. London: European Network of Centres for Pharmacoepidemiology and Pharmacovigilance) [19]. |
Table 1

ENCePP, European Network of Centres for Pharmacoepidemiology and Pharmacovigilance; CeVEAS, Centre for the Evaluation of Effectiveness of Health Care; RTI, Research Triangle Institute; EMA, European Medicines Agency; HMA, Heads of Medicines Agencies; CHMP, Committee for Medicinal Products for Human Use; AGES, Austrian Agency for Health and Food Safety; COMP, Committee for Orphan Medicinal Products; INFARMED, Instituto Nacional da Farmácia e do Medicamento Parque da Saúde de Lisboa; PRAC, Pharmacovigilance Risk Assessment Committee; PCWP, EMA Human Scientific Committees' Working Party with Patients' and Consumers' Organisations; ISPE, International Society for Pharmacoepidemiology; ISoP, International Society of Pharmacovigilance; EFPIA, European Federation of Pharmaceutical Industries and Associations.
From European Network of Centres for Pharmacoepidemiology and Pharmacovigilance. ENCePP steering group [Internet]. London: European Network of Centres for Pharmacoepidemiology and Pharmacovigilance [18].




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download