Abstract
The decision making for reimbursement has become more difficult than before because of the high cost innovative new technologies and limitation of healthcare resources. Evidence-based healthcare system has been introduced in Korea since 2007. Not infrequently, however, decision makers have been confronted with uncertainties caused by lack of information related to comparative effectiveness, real world outcomes, off-label drug use, combination therapy and cost-effectiveness. Under these circumstances, the decision making of whether or not to reimburse is inadequate because undesirable results may be induced. Managed entry agreement has been introduced globally. This kind of decision is conditional and linked with monitoring and following the results and adjusting the policy according to the results. Especially access with evidence development is a form of prospective data correction with conditional coverage in the case of existing uncertainties of effectiveness and safety. In Korea, there have been several examples including off-label drug use in oncologic drugs, new healthcare intervention and re-evaluation of existing drugs. Even though there has been some conflict and confusion because of inadequate systematization, it is strongly recommended to establish the conditional decision making system in Korea since this is a unique answer to manage the uncertainty in maintaining development of health science, keeping patients' right and using healthcare resources rationally.
Figures and Tables
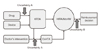 | Figure 2Stage of regulation and decision process for adoption of new health technology and need for conditional decision with evidence generation. KFDA, Korean Food and Drug Administration; CnHTA, Committee for New Health Technology Assessment; HIRA, Health Insurance Review and Assessment Service; MoHW, Ministry of Health and Welfare, Korea. |
References
1. Park SH, Lee SM. Evidence-based decision-making and health technology assessment in South Korea. Value Health. 2008. 11:Suppl 1. S163–S164.

2. Lee SM. Evidence-based healthcare: seeking for the broken virtuous circle. J Korean Med Assoc. 2009. 52:532–535.

3. Stafinski T, McCabe CJ, Menon D. Funding the unfundable: mechanisms for managing uncertainty in decisions on the introduction of new and innovative technologies into healthcare systems. Pharmacoeconomics. 2010. 28:113–142.
4. Klemp M, Fronsdal KB, Facey K. HTAi Policy Forum. What principles should govern the use of managed entry agreements? Int J Technol Assess Health Care. 2011. 27:77–83.

5. Eden J, Wheatley B, McNeil B, Sox H. Knowing what works in health care: a roadmap for the nation. 2008. Washington, DC: The National Academies Press;280.
6. Park S. Risk-sharing agreement on the pricing and reimbursement of new drugs. Korean J Health Econ Policy. 2010. 16:125–153.
7. Organisation for Economic Co-operation and Development (OECD). Pharmaceutical pricing policies in a global market. 2008. Paris: OECD;215.
8. Park S, Park EJ, Ko SJ. Policy measures to enhance access to drugs for rare diseases. 2010. Seoul: Korea Institute for Health and Social Affairs.
9. Olsen LA, Aisner D, McGinnis JM. The learning healthcare system: workshop summary. 2007. Washington, DC: National Academies Press;374.
10. Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D. CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006. 355:2085–2098.

11. Rubin RJ, Mendelson DN. Translating guidelines into policy. Clin J Am Soc Nephrol. 2007. 2:209–210.

12. Green Park Collaborative (GPC) [Internet]. Center for Medical Technology Policy. c2008. cited 2011 Oct 20. Baltimore, MD: Center for Medical Technology Policy;Available from: http://www.cmtpnet.org/gpc.
13. Carino T, Williams RD 2nd, Colbert AM, Bridger P. Medicare's coverage of colorectal cancer drugs: a case study in evidence development and policy. Health Aff (Millwood). 2006. 25:1231–1239.

14. Chalkidou K, Hoy A, Littlejohns P. Making a decision to wait for more evidence: when the National Institute for Health and Clinical Excellence recommends a technology only in the context of research. J R Soc Med. 2007. 100:453–460.

15. Beta interferon and glatiramer acetate for the treatment of multiple sclerosis [Internet]. National Institute for Clinical Excellence. 2002. cited 2011 Oct 20. London: National Institute for Clinical Excellence;Available from: http://www.nice.org.uk/nicemedia/live/11441/32290/32290.pdf.
16. Lexchin J. Coverage with evidence development for pharmaceuticals: a policy in evolution? Int J Health Serv. 2011. 41:337–354.

17. McCabe CJ, Stafinski T, Edlin R, Menon D. Banff AED Summit. Access with evidence development schemes: a framework for description and evaluation. Pharmacoeconomics. 2010. 28:143–152.
18. Boggild M, Palace J, Barton P, Ben-Shlomo Y, Bregenzer T, Dobson C, Gray R. Multiple sclerosis risk sharing scheme: two year results of clinical cohort study with historical comparator. BMJ. 2009. 339:b4677.

20. Wlodarczyk JH, Cleland LG, Keogh AM, McNeil KD, Perl K, Weintraub RG, Williams TJ. Public funding of bosentan for the treatment of pulmonary artery hypertension in Australia: cost effectiveness and risk sharing. Pharmacoeconomics. 2006. 24:903–915.

21. Trueman P, Grainger DL, Downs KE. Coverage with Evidence Development: applications and issues. Int J Technol Assess Health Care. 2010. 26:79–85.

22. Persson U, Willis M, Odegaard K. A case study of ex ante, value-based price and reimbursement decision-making: TLV and rimonabant in Sweden. Eur J Health Econ. 2010. 11:195–203.

23. Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G, Wood DE. National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003. 348:2059–2073.

24. Mohr PE, Tunis SR. Access with evidence development: the US experience. Pharmacoeconomics. 2010. 28:153–162.
25. Tu JV, Bowen J, Chiu M, Ko DT, Austin PC, He Y, Hopkins R, Tarride JE, Blackhouse G, Lazzam C, Cohen EA, Goeree R. Effectiveness and safety of drug-eluting stents in Ontario. N Engl J Med. 2007. 357:1393–1402.

26. Park BJ, Heo DS, Ahn HS, Lee SM, Lee YH, Kim SY, Kim SS, Han SG, Bae EY, Park DA. Evidence-based healthcare. 2009. Seoul: Korean Medical Book Publisher;364.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download