Abstract
Rheumatoid arthritis (RA) is a chronic and progressive inflammatory disorder, characterized by synovitis and severe joint destruction. Many mechanisms are considered to be implicated in the development and progression of the disease. It may be important to understand differences in the pathogenesis of RA at various stages of its process. Early autoimmune changes begin before the onset of clinical arthritis. During this period, various autoantibodies such as rheumatoid factor and anticyclic citrullinated peptide antibody can be produced by the interaction between B and T cell activated by aberrant immune responses, triggered by external or self antigens. This is followed by a local inflammatory transitional phase, in which complex biochemical processes are involved in molecular and structural changes of the joint. The primary inflammatory site is the synovium. Synovial infiltration with mononuclear cells, especially CD4+ T cells, macrophages, and B cells leads to an articular, pathologic phase. In this phase, proinflammatory cytokines including tumor necrosis factor-α, interleukin-1β, and interleukin-6 as well as inflammatory mediators such as prostaglandin E2 and proteases can be produced by various cell to cell interaction occuring in the synovium, which may finally result in the destruction of synovium, cartilage and bone. Although the pathogenesis of RA is intricate and remains unclear, understanding and identifying its pathogenesis is important in revealing the appropriate therapeutic target that may lead to significant clinical benefits.
Figures and Tables
Figure 1
The role of Th17 cell in the pathogenesis of rheumatoid arthritis. IL-17 produced by Th17 cell influences various kinds of cells and is involved in the development of inflammation, cartilage and bone loss, which are characteristics of rheumatoid arthritis. Abbreviations: MCP-1, Monocyte chemotactic peptide-1; GRO-α, Growth regulated protein-α; MMPs, Matrix metalloproteinases; NO, Nitric oxide; RANKL, Receptor Activator for Nuclear Factor κB Ligand.
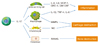
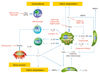
Figure 2
Cytokine network in rheumatoid arthritis as a background for anticytokine therapy. Abbreviations: Blys, B lymphocyte stimulator; APRIL, A proliferation-inducing ligand; MMP, Matrix metalloproteinase; RANKL, Receptor Activator for Nuclear Factor κB Ligand; RANTES, Regulated on activation, normal T cell expressed and secreted
References
1. Leipe J, Grunke M, Dechant C, Reindl C, Kerzendorf U, Schulze-Koops H, Skapenko A. Role of Th17 cells in autoimmune arthritis. Arthritis Rheum. 2010. 62:2876–2885.
2. Furuzawa-Carballeda J, Vargas-Rojas MI, Cabral AR. Autoimmune inflammation from the Th17 perspective. Autoimmun Rev. 2007. 6:169–175.

3. Nistala K, Moncrieffe H, Newton KR, Varsani H, Hunter P, Wedderburn LR. Interleukin-17-producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbers. Arthritis Rheum. 2008. 58:875–887.

4. Kim HY, Kim WU, Cho ML, Lee SK, Youn J, Kim SI, Yoo WH, Park JH, Min JK, Lee SH, Park SH, Cho CS. Enhanced T cell proliferative response to type II collagen and synthetic peptide CII (255-274) in patients with rheumatoid arthritis. Arthritis Rheum. 1999. 42:2085–2093.

5. Park SH, Min DJ, Cho ML, Kim WU, Youn J, Park W, Cho CS, Kim HY. Shift toward T helper 1 cytokines by type II collagen-reactive T cells in patients with rheumatoid arthritis. Arthritis Rheum. 2001. 44:561–569.

6. Cho ML, Yoon CH, Hwang SY, Park MK, Min SY, Lee SH, Park SH, Kim HY. Effector function of type II collagen-stimulated T cells from rheumatoid arthritis patients: cross-talk between T cells and synovial fibroblasts. Arthritis Rheum. 2004. 50:776–784.

7. Min DJ, Cho ML, Lee SH, Min SY, Kim WU, Min JK, Park SH, Cho CS, Kim HY. Augmented production of chemokines by the interaction of type II collagen-reactive T cells with rheumatoid synovial fibroblasts. Arthritis Rheum. 2004. 50:1146–1155.

8. De Rycke L, Nicholas AP, Cantaert T, Kruithof E, Echols JD, Vandekerckhove B, Veys EM, De Keyser F, Baeten D. Synovial intracellular citrullinated proteins colocalizing with peptidyl arginine deiminase as pathophysiologically relevant antigenic determinants of rheumatoid arthritis-specific humoral autoimmunity. Arthritis Rheum. 2005. 52:2323–2330.

9. Chang X, Yamada R, Suzuki A, Sawada T, Yoshino S, Tokuhiro S, Yamamoto K. Localization of peptidylarginine deiminase 4 (PADI4) and citrullinated protein in synovial tissue of rheumatoid arthritis. Rheumatology (Oxford). 2005. 44:40–50.

10. Cho ML, Kang JW, Moon YM, Nam HJ, Jhun JY, Heo SB, Jin HT, Min SY, Ju JH, Park KS, Cho YG, Yoon CH, Park SH, Sung YC, Kim HY. STAT3 and NF-kappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J Immunol. 2006. 176:5652–5661.

11. Kim HR, Cho ML, Kim KW, Juhn JY, Hwang SY, Yoon CH, Park SH, Lee SH, Kim HY. Up-regulation of IL-23p19 expression in rheumatoid arthritis synovial fibroblasts by IL-17 through PI3-kinase-, NF-kappaB- and p38 MAPK-dependent signalling pathways. Rheumatology (Oxford). 2007. 46:57–64.

12. Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004. 350:2572–2581.

13. Emery P, Fleischmann R, Filipowicz-Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, Racewicz AJ, van Vollenhoven RF, Li NF, Agarwal S, Hessey EW, Shaw TM. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 2006. 54:1390–1400.

14. Cush JJ, Splawski JB, Thomas R, McFarlin JE, Schulze-Koops H, Davis LS, Fujita K, Lipsky PE. Elevated interleukin-10 levels in patients with rheumatoid arthritis. Arthritis Rheum. 1995. 38:96–104.

15. He X, Goronzy JJ, Weyand CM. The repertoire of rheumatoid factor-producing B cells in normal subjects and patients with rheumatoid arthritis. Arthritis Rheum. 1993. 36:1061–1069.

16. Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH. Rheumatology. 2007. 4 ed. MOSBY;811–866.
17. McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007. 7:429–442.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download