Abstract
The number of clinical trials sponsored by global pharmaceutical companies performed in countries other than the U.S. and Western Europe has been steadily increasing over the past decade. Among those emerging countries, Korea deserves attention for its rapid growth in the number of trials and sites. As of 2009, Korea was ranked the tenth country in the number of clinical trials registered at http://clinicaltrials.gov. This is remarkable growth given that it was not included in the top 30 countries in 2005. High population density, qualified medical professionals, regulatory changes including Investigational New Drug-New Drug Application (IND/NDA) separation, acceptance of International Conferences on Harmonization-Good Clinical Practices (ICH-GCP) by Korea Food&Drug Administration (KFDA), and governmental policies to boost clinical trials were the most influential factors that caused such an outstanding achievement. The Korean National Enterprise for Clinical Trials (KoNECT), an organization founded to lead initiatives to improve the milieu for clinical trials, has been playing a pivotal role in the steering of 15 regional clinical centers designated by the government. Based upon improvements in infrastructure so far, diversity in therapeutic areas and the proportion of early phase trials are expected to grow. Korea has grown to be one of the major countries in the clinical trial market, which was made possible by the cooperation of industry, academia and government. Further investment and efforts to solve current challenges will allow such growth to continue into the next decade.
Figures and Tables
Figure 3
Numbers (A) and densities (B, institutions per 100,000 residents) of clinical trial institutions by region.

Table 2
Number of clinical trials (world) classified by phases and their annual growth rates in 2008 and 2009 (Dialog Corporation[7])

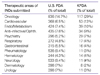
Table 3
Company-sponsored clinical trials-North America, Europe and Korea in 2009 (www.clinicaltrials.gov)[7]

References
1. Karlberg J. Industry Sponsored Clinical Trials in South Korea. Clin Trial Magnifier. 2008. 1:179–192.
2. KFDA Press release. 2010. 07. 20.
3. IMS Press release, IMS Forecasts Global Pharmaceutical Market Growth of 5-8% Annually through 2014; Maintains Expectations of 4-6% Growth in 2010. 2010. 04. 20.
4. IMS Press release, IMS Forecasts Global Pharmaceutical Market Growth of 4-6% in 2010; Predicts 4-7% Expansion Through 2013. 2009. 10. 07.
5. KFDA Press release. 2010. 06. 10.
6. Yoon SH, Moon JE, Oh SJ, Choi JH, Kim YI. Research Development Status of Biosimilar Drugs. J Cosmet Public Health. 2009. 5:108–113.
7. KFDA Press release. 2010. 02. 09.
8. The Hub of Clinical Trial. The Korean Hospital News. 2010. 07. 08.
9. MOWH Press release. 2010. 07. 26.
10. Accredited Clinical Trial Institutions, KFDA. 2010. 06. 24.
11. FERCAP-SIDCER Recognized ECs/IRBs. Available from:
http://www.fercap-sidcer.org/recognized.php.
12. AAHRPP accredited organizations. Available from: http://www.aahrpp.org/www.aspx?PageID=11&Country=KR#grid.
13. Kim DY, Kwon OD, Kim SG, Shin IH. Recognitional study about patients and caregivers' understanding of clinical trial. J Korean Continence Soc. 2008. 12:48–57.

14. Moon H, Kim HK, Lee KS, Kang BK, Song HH. A Survey of the Cognition on Clinical Trial and Informed Consent of Cancer Patients and Their Families. J Korean Soc Clin Pharmacol Ther. 1995. 3:141–150.

15. World health statistics 2010. World Health Organization. Available from: http://www.who.int/whosis/whostat/2010.
16. Mathieu M. Strategy Report to Enhance Korea's Global Competitiveness Through the Detailed and Professional Analysis of Global and Asia. Clinical Trial Environments. 57–64.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download