Abstract
Epilepsy associated with brain tumors (EABT) is a multi-faceted disease that both oncological and epileptological concerns should be taken into consideration. Usually, it is characterized by chronic drug-resistant epilepsy with a low-grade brain tumor in the cerebrum. However, the distinction of typical EABT and simple brain tumors with short-term epilepsy is obscure. We need a working formulation based on the patient? s burden in both oncological and epileptological aspects. The diagnosis of EABT is straightforward, but the treatment should be more complex. Medical treatment with anticonvulsants aloneseems tobe anoutdated remedy for EABT because of the risk of tumor growth and malignant progression in some patients as well as the expected favorable seizure control after surgery. Surgical treatment of EABT has resulted in seizure-free state in about 80% of patients. Complete resection of the tumor is an important prognostic factor in seizure control and probably also in tumor control. Recently, many authors emphasized a lesion-directed surgery aimed at a complete tumor removal in EABT. However, in some patients, especially in patients with dual pathology, electrophysiological studies have to be thoroughly applied. For the treatment of EABT in the temporal lobe, more sophisticated surgical strategy is required. A lesionectomy saving the uninterrupted hippocampus could be applied for selected patients. Further research is strongly needed for better understanding and treatment of EABT and low-grade glioma.
Go to : 
REFERENCES
1. Feindel W. Osler vindicated: glioma of the leg center with Jacksonian epilepsy; removal and cure, with a 50-year followup. Historical vignette. J Neurosurg. 2009; 111:293–300.
2. Bliss M. Harvey Cushing: A life in surgery. New York: Oxford University Press, Inc. 2005. 84–130.
3. Teive HA, Germiniani FM, Cardoso AB, de Paola L, Werneck LC. The uncinated crisis of George Gershwin. Arq Neuropsi-quiatr. 2002; 60:505–508.

4. Carp L. George Gershwin-illustrious American composer: his fatal glioblastoma. Am J Surg Pathol. 1979; 3:473–478.
5. Silverstein A. The brain tumor of George Gershwin and the legs of Cole Porter. Semin Neurol. 1999; 19:3–9.
6. van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007; 6:421–430.

7. Wolf HK, Roos D, Blumcke I, Pietsch T, Wiestler OD. Perilesional neurochemical changes in focal epilepsies. Acta Neuropathol. 1996; 91:376–384.

9. Olafsson E, Ludvigsson P, Gudmundsson G, Hesdorffer D, Kjartansson O, Hauser WA. Incidence of unprovoked seizures and epilepsy in Iceland and assessment of the epilepsy syndrome classification: a prospective study. Lancet Neurol. 2005; 4:627–634.

10. Clusmann H, Schramm J, Kral T, Helmstaedter C, Ostertun B, Fimmers R, Haun D, Elger CE. Prognostic factors and outcome after different types of resection for temporal lobe epilepsy. J Neurosurg. 2002; 97:1131–1141.

11. Spooner CG, Berkovic SF, Mitchell LA, Wrennall JA, Harvey AS. New-onset temporal lobe epilepsy in children: lesion on MRI predicts poor seizure outcome. Neurology. 2006; 67:2147–2153.

12. Luyken C, Blumcke I, Fimmers R, Urbach H, Elger CE, Wiestler OD, Schramm J. The spectrum of longterm epilepsy-associated tumors: longterm seizure and tumor outcome and neurosurgical aspects. Epilepsia. 2003; 44:822–830.

13. Phi JH, Chung CK. Brain tumors in the mesial temporal lobe: longterm oncological outcome. Neurosurg Focus. 2009; 27:E5.

14. Blumcke I, Wiestler OD. Gangliogliomas: an intriguing tumor entity associated with focal epilepsies. J Neuropathol Exp Neurol. 2002; 61:575–584.

15. Nolan MA, Sakuta R, Chuang N, Otsubo H, Rutka JT, Snead OC 3rd, Hawkins CE, Weiss SK. Dysembryoplastic neuroepithelial tumors in childhood: longterm outcome and prognostic features. Neurology. 2004; 62:2270–2276.

16. Phi JH, Kim SK, Cho BK, Lee SY, Park SY, Park SJ, Lee SK, Kim KJ, Chung CK. Longterm surgical outcomes of temporal lobe epilepsy associated with low-grade brain tumors. Cancer. 2009; 115:5771–5779.

17. Daumas-Duport C, Scheithauer BW, Chodkiewicz JP, Laws ER Jr, Vedrenne C. Dysembryoplastic neuroepithelial tumor: a surgically curable tumor of young patients with intractable partial seizures. Report of thirty-nine cases. Neurosurgery. 1988; 23:545–556.
18. Takahashi A, Hong SC, Seo DW, Hong SB, Lee M, Suh YL. Frequent association of cortical dysplasia in dysembryoplastic neuroepithelial tumor treated by epilepsy surgery. Surg Neurol. 2005; 64:419–427.

19. Sakuta R, Otsubo H, Nolan MA, Weiss SK, Hawkins C, Rutka JT, Chuang NA, Chuang SH, Snead OC 3rd. Recurrent intractable seizures in children with cortical dysplasia adjacent to dysembryoplastic neuroepithelial tumor. J Child Neurol. 2005; 20:377–384.

20. Barkovich AJ, Kuzniecky RI, Jackson GD, Guerrini R, Dobyns WB. A developmental and genetic classification for malformations of cortical development. Neurology. 2005; 65:1873–1887.

21. Blumcke I, Lobach M, Wolf HK, Wiestler OD. Evidence for developmental precursor lesions in epilepsy-associated glio-neuronal tumors. Microsc Res Tech. 1999; 46:53–58.
22. Bartolomei JC, Christopher S, Vives K, Spencer DD, Piepmeier JM. Low-grade gliomas of chronic epilepsy: a distinct clinical and pathological entity. J Neurooncol. 1997; 34:79–84.
23. Piepmeier J, Christopher S, Spencer D, Byrne T, Kim J, Knisel JP, Lacy J, Tsukerman L, Makuch R. Variations in the natural history and survival of patients with supratentorial low-grade astrocytomas. Neurosurgery. 1996; 38:872–878.
24. Piepmeier JM, Christopher S. Low-grade gliomas: introduction and overview. J Neurooncol. 1997; 34:1–3.
25. Blumcke I, Luyken C, Urbach H, Schramm J, Wiestler OD. An isomorphic subtype of longterm epilepsy-associated astrocytomas associated with benign prognosis. Acta Neuropathol. 2004; 107:381–388.
26. Schramm J, Luyken C, Urbach H, Fimmers R, Blumcke I. Evidence for a clinically distinct new subtype of grade II astrocytomas in patients with longterm epilepsy. Neurosurgery. 2004; 55:340–347.

27. Eriksson SH, Nordborg C, Rydenhag B, Malmgren K. Parenchymal lesions in pharmacoresistant temporal lobe epilepsy: dual and multiple pathology. Acta Neurol Scand. 2005; 112:151–156.

28. Salanova V, Markand O, Worth R. Temporal lobe epilepsy: analysis of patients with dual pathology. Acta Neurol Scand. 2004; 109:126–131.

29. Stephen LJ, Kwan P, Brodie MJ. Does the cause of localisation-related epilepsy influence the response to antiepileptic drug treatment? Epilepsia. 2001; 42:357–362.
30. Perry A. Pathology of low-grade gliomas: an update of emerging concepts. Neuro Oncol. 2003; 5:168–178.

31. Majores M, von Lehe M, Fassunke J, Schramm J, Becker AJ, Simon M. Tumor recurrence and malignant progression of gangliogliomas. Cancer. 2008; 113:3355–3363.

32. Luyken C, Blumcke I, Fimmers R, Urbach H, Wiestler OD, Schramm J. Supratentorial gangliogliomas: histopathologic grading and tumor recurrence in 184 patients with a median followup of 8 years. Cancer. 2004; 101:146–155.

34. Klein M, Engelberts NH, van der Ploeg HM, Kasteleijn-Nolst Trenite DG, Aaronson NK, Taphoorn MJ, Baaijen H, Vandertop WP, Muller M, Postma TJ, Heimans JJ. Epilepsy in low-grade gliomas: the impact on cognitive function and quality of life. Ann Neurol. 2003; 54:514–520.

35. Zaatreh MM, Firlik KS, Spencer DD, Spencer SS. Temporal lobe tumoral epilepsy: characteristics and predictors of surgical outcome. Neurology. 2003; 61:636–641.
36. Cohen-Gadol AA, Ozduman K, Bronen RA, Kim JH, Spencer DD. Longterm outcome after epilepsy surgery for focal cortical dysplasia. J Neurosurg. 2004; 101:55–65.

37. Hader WJ, Mackay M, Otsubo H, Chitoku S, Weiss S, Becker L, Sned OC 3rd, Rutka JT. Cortical dysplastic lesions in children with intractable epilepsy: role of complete resection. J Neurosurg. 2004; 100:110–117.

38. Duffau H. Surgery of low-grade gliomas: towards a ?functional neurooncology? Curr Opin Oncol. 2009; 21:543–549.

39. Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, Tihan T, Vandenberg S, McDermott MW, Berger MS. Role of extent of resection in the longterm outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008; 26:1338–1345.

40. McGirt MJ, Chaichana KL, Attenello FJ, Weingart JD, Than K, Burger PC, Olivi A, Brem H, Wuinones-Hinojosa A. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008; 63:700–707.

41. Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008; 358:18–27.

42. Lombardi D, Marsh R, de Tribolet N. Low grade glioma in intractable epilepsy: lesionectomy versus epilepsy surgery. Acta Neurochir Suppl. 1997; 68:70–74.

43. Jooma R, Yeh HS, Privitera MD, Gartner M. Lesionectomy versus electrophysiologically guided resection for temporal lobe tumors manifesting with complex partial seizures. J Neurosurg. 1995; 83:231–236.

44. Sugano H, Shimizu H, Sunaga S. Efficacy of intraoperative electrocorticography for assessing seizure outcomes in intractable epilepsy patients with temporal-lobe-mass lesions. Seizure. 2007; 16:120–127.

45. Giulioni M, Galassi E, Zucchelli M, Volpi L. Seizure outcome of lesionectomy in glioneuronal tumors associated with epilepsy in children. J Neurosurg. 2005; 102:288–293.

46. Kim SK, Wang KC, Hwang YS, Kim KJ, Cho BK. Intractable epilepsy associated with brain tumors in children: surgical modality and outcome. Childs Nerv Syst. 2001; 17:445–452.

47. Minkin K, Klein O, Mancini J, Lena G. Surgical strategies and seizure control in pediatric patients with dysembryoplastic neuroepithelial tumors: a single-institution experience. J Neurosurg Pediatrics. 2008; 1:206–210.

48. Chang EF, Potts MB, Keles GE, Lamborn KR, Chang SM, Barbaro NM, Berger MS. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. 2008; 108:227–235.

49. Cataltepe O, Turanli G, Yalnizoglu D, Topcu M, Akalan N. Surgical management of temporal lobe tumor-related epilepsy in children. J Neurosurg. 2005; 102:280–287.
Go to : 
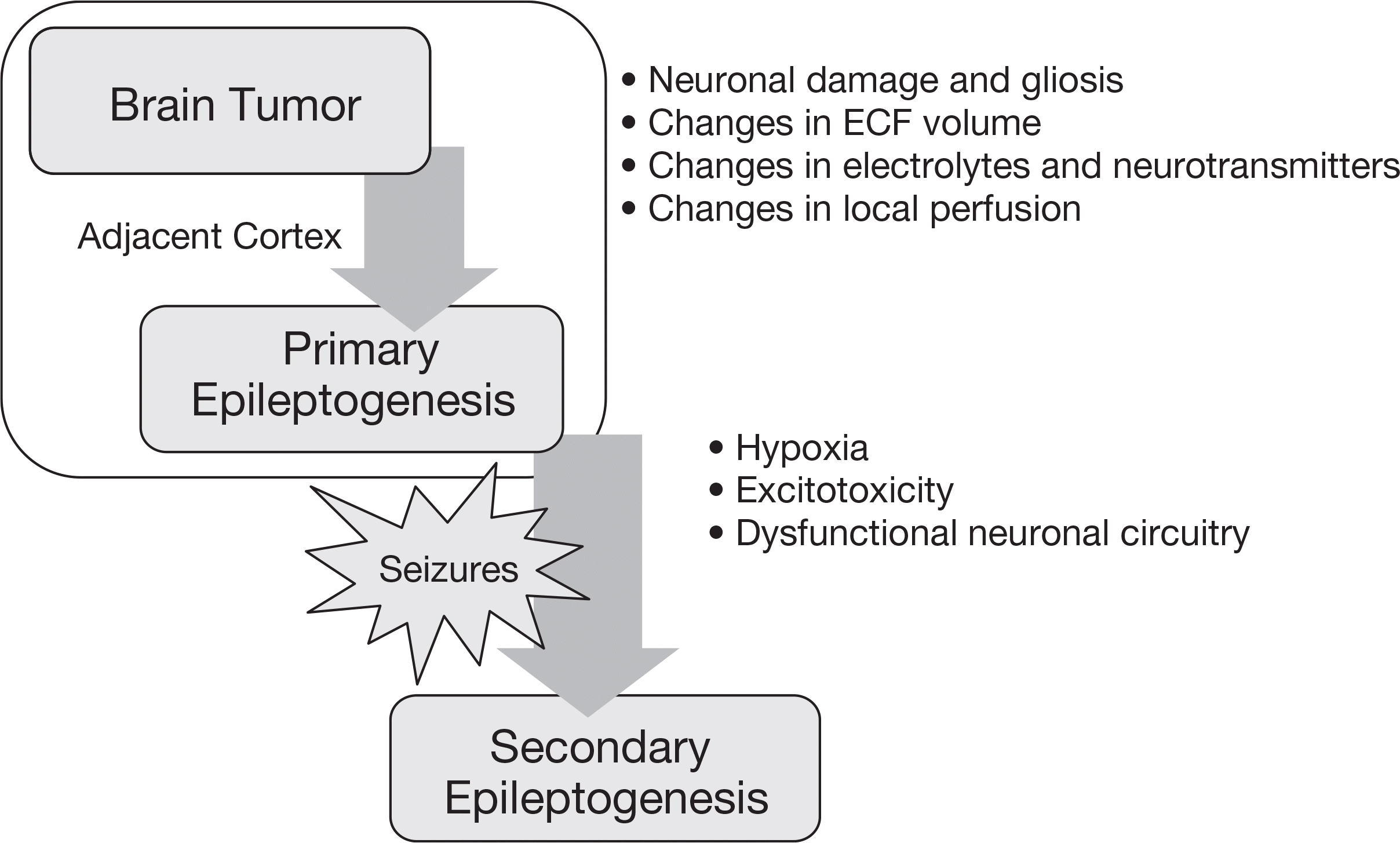 | Figure 1.A diagram depicting the epileptogenesis in the presence of a brain tumor and disease progression. Dual pathology can be attributed to secondary epileptogenesis. |
 | Figure 2.MR images of a 36-year-old female with 8-year-history of complex partial seizures. (A)A 2cm-sized tumor (arrow) is observed in right lateral temporal lobe in a T2-weighted image. (B)In a FLAIR image, ipsilateral hippocampus (arrow) is smaller than the counterpart and its signal intensity is increased, which are typical of hippocampal sclerosis. The patient had dual pathology. |
 | Figure 3.Preoperative and postoperative T2-weighted MR images of a 22-year-old male with 2-year-history of complex partial seizures. (A)A 1cm-sized tumor (arrow) is observed in left amygdaloid body in a preoperative image. (B)The amygdaloid body and the tumor were completely removed via a transsylvivan approach. The uninvolved hippocampus was preserved. The intact hippocampus (arrow) is observed in a postoperative image. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download