Abstract
Figures and Tables
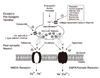 | Figure 1An excitatory synapse. the putative major sites of action of various AEds.
MDA: N-methyl-D-asparate, AMPA: a-amino-3-hydroxy-4-isoxazo-lepropionic acid (2).
|
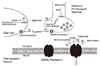 | Figure 2An inhibitory synapse. the putative major sites of action of various AEds.
GABA: γ-aminobutyric acid, GABA-T, GABA transaminase, GAD: glutamic acid decarboxylase (2).
|
 | Figure 3Plasma concentration of a drug following repeated oral drug administration (4) as a function of interval of administration measured as multiples of elimination half-life. |
 | Figure 4Relationship between serum drug concentration (ordinate) and drug dose (abscissa) for a drug observing (B) first-order kinetics (linear) and (A, C) zero-order kinetics (4) A, PHT, ZNS; B, FBM, ESM, PB, TPM, VPA,OXC, LEV, ZNS; C, LTG, VPA. |
Table 6

↔, no interaction, F-1, decrease in progestin; F-2, increase in wafarin; G-1and G-2, decrease in GBP L-1, decrease in LTG; L-2, increase in LTG; O-1, decrease in cyclosporine; O-2, decrease in ethinyl estradiol; T-1, decrease in digoxin; T-2, decrease in ethinyl estradiol; T-3, increase in haloperidol.
Table 10

* based on less than class I and class II evidence (5).
BECTS, benign epilepsy of childhood with centrotemporal spikes; CJAE, childhood and juvenile absence epilepsy; JME, juvenile myoclonic epilepsy; GTC, generalized tonic-clonic seizures.
Table 12

BECT, BECTS, benign epilepsy of childhood with centrotemporal spikes.
* Pediatric Expert Consensus survey. Drugs rated as treatment of choice listed
† International League Against Epilepsy, Recommendations listed according to levels of evidence supporting the efficacy options. Level A, B, C (French JA, Kanner AM, Bautista J, et al. Efficacy and tolerability of the new antiepileptic drug I: treatment of new onset epilepsy. Neurology 2004; 62: 1252 -1260)
‡ SIGN: scottish intercollegiate guideline network. Diagnosis and manegement of epilepsies in children and young people; A national clinical guideline Edinburgh, SIGN: MArch 2005. (Copies available at: http://www.sign.ac.uk/pdf/sign81.pdf)
§ National Institute for Clincal Excellence, Technology Appraisal Guidance 79. Newer drugs for epilepsy in children (www.nice.org/uk/TA079 guidance) and Clinical guidance 20. The epilepsies: The diagnosis and mangement of the epilepsies in adults and children in primary and secondary care, October 2004 (www.nice.org/uk/CG020NICE guideline).
∥ FDA approval for each selzures type or epilepsy syndrome.
Table 18

+, limited; ++, moderate; +++. significant.
JME, juvenile myoclonic epilepsy; LGS, Lennox-Gastaut syndrome; MAE, myoclonic astatic epilepsy; BECTS, benign epilepsy of childhood with centrotemporal spikes; SMEI, severe myoclonic epilepsy of infancy; LKS, Landau-Kleffner syndrome; ESES, electrical status epilepticus of sleep.




 PDF
PDF ePub
ePub Citation
Citation Print
Print
















 XML Download
XML Download