Abstract
Hypersensitivity pneumonitis (HP), also known as extrinsic allergic alveolitis, is an immunologically mediated granulomatous, inflammatory disease of the lungs caused by repeated inhalation of various antigens. HP may occur in acute, subacute, or chronic forms. Chronic HP may be progressive, irreversible, and evolve to fibrotic interstitial lung disease. The diagnosis of HP can be made from a combination of clinical, laboratory, radiologic, and pathologic findings. A careful environmental and occupational history and establishment of exposure to a known inciting antigen are key factors in making the diagnosis of HP. Serum precipitating antibodies, bronchoalveolar lavage, and lung biopsy may be helpful in making the diagnosis. The pathology of HP is characterized by interstitial lymphocytic infiltration, poorlyformed noncaseating granulomas, cellular bronchiolitis, and fibrosis. In the pathogenesis of HP, recent studies showed that both type III and type IV hypersensitivity reactions are involved and are mediated by immune complexes and Th1 T cells, respectively. IFN-γ is essential for the development of HP, and IL-10 appears to modulate the severity of the disease. TNF-α and TGF-β have been implicated in development of the pulmonary fibrosis that is seen in chronic HP. Avoidance of organic antigen exposure is the most important factor for the management of HP. There is often an apparent beneficial response to corticosteroids in the cases of severe acute and subacute HP, and for chronic HP that is severe or progressive.
Figures and Tables
 | Figure 1Chest X-ray of acute hypersensitivity pneumonitis.
Chest X-ray showed some reticulonodular densities in both upper lobes.
|
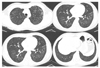 | Figure 2High-resolution computed tomography scans of acute hypersensitivity pneumonitis showed discrete ground glass attenuation and tiny, ill-defined centrilobular nodules on upper and lower lung field. |
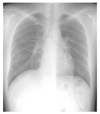 | Figure 3After recurrent exposure to antigen, chest X-ray showed diffuse poorly-defined small nodular densities and diffuse ground glass opacities in subacute patient with hypersensitivity pneumonitis. |
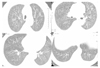 | Figure 4High-resolution computed tomography scans of subacute hypersensitivity pneumonitis showed discrete ground glass attenuatiuon and tiny, ill-defined centrilobular nodules, predominant on upper lung field. |
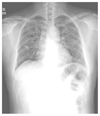 | Figure 5Chest X-ray of chronic hypersensitivity pneumonitis.
Chest X-ray showed coarse rericular opacities with diffusely scattered patchy ground glass opacities in both lung fields.
|
 | Figure 6(A) High-resolution computed tomography scans of chronic hypersensitivity pneumonitis showed patchy areas of lung fibrosis, mainly peribronchiolar regions, and traction bronchiectasis or bronchiolectasis, slightly dominant on the upper lung field. Lobular air trappings were also seen in multifocal areas.
(B) End-stage chronic hypersensitivity pneumonitis.
|
 | Figure 7Pathologic findings of subacute hypersensitivity pneumonitis. Lymphocyte infiltration of the respiratory bronchioles and alveolar walls with ill-defined granuloma (H & E × 100). |
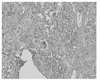 | Figure 8Pathologic findings of chronic hypersensitivity pneumonitis. Chronic interstitial pneumonia pattern with illdefined granuloma and focal fibroblastic proliferation (H & E × 100). |
Table 4
Histologic differential diagnosis of hypersensitivity pneumonitis

HP: hypersensitivity pneumonitis, LIP: lymphoid interstitial pneumonia, NSIP: nonspecific interstitial pneumonia, UIP: usual interstitial pneumonia, Modified from Takemura, et al (17).
References
1. Selman M. Schwarz MI, King TE, editors. Hypersensitivity pneumonitis. Interstitial lung disease. 2003. 4th ed. London: BC Decker Inc;452–484.

3. Selman M. Hypersensitivity pneumonitis: a multifaceted deceiving disorder. Clin Chest Med. 2004. 25:531–547.

4. McSharry C, Anderson K, Bourke SJ, Boyd G. Takes your breath away-the immunology of allergic alveolitis. Clin Exp Immunol. 2002. 128:3–9.
5. Bourke SJ, Dalphin JC, Boyd G, McSharry C, Baldwin CI, Calvert JE. Hypersensitivity pneumonitis: current concepts. Eur Respir J. 2001. 18:S. 81–92.
6. Patel AM, Ryu JH, Reed CE. Hypersensitivity pneumonitis: current concepts and future questions. J Allergy Clin Immunol. 2001. 108:661–670.

7. Schuyler M, Gott K, Cherne A. Mediators of hypersensitivity pneumonitis. J Lab Clin Med. 2000. 136:29–38.

8. Yamasaki H, Ando M, Brazer W, Center DM, Cruilshank WW. Polarized type I cytokine profile in bronchoalveolar lavage T cells in patients with hypersensitivity pneumonitis. J Immunol. 1999. 163:3516–3523.
9. Suga M, Yamasaki H, Nakagawa K, Kohroqi H, Ando M. Mechanisms accounting for granulomatous responses in hypersensitivity pneumonitis. Sarcoidosis Vasc Diffuse Lung Dis. 1997. 14:131–138.
11. Girard M, Israel-Assayag E, Cormier Y. Pathogenesis of hypersensitivity pneumonitis. Curr Opin Allergy Clin Immunol. 2004. 4:93–98.

12. Camarena A, Juarez A, Mejia M, Estrada A, Carrillo G, Falfán R, Zuñiga J, Navarro C, Granados J, Selman M. Major histocompatibility complex and tumor necrosis factor-a polymorphism in pigeon breeder's disease. Am J Respir Crit Care Med. 2001. 163:1528–1533.

13. Glazer CS, Rose CS, Lynch DA. Clinical and radiologic manifestations of hypersensitivity pneumonitis. J Thorac Imaging. 2002. 17:261–272.

14. Silva CI, Churg A, Muller NL. Hypersensitivity pneumonitis: spectrum of high-resolution CT and pathologic findings. AJR. 2007. 188:334–344.

15. Churg A, Muller NL, Flint J, Wright JL. Chronic hypersensitivity pneumonitis. Am J Surg Pathol. 2006. 30:201–208.

16. Lacasse Y, Selman M, Costabel U, Dalphin JC, Ando M, Morell F, Erkinjuntti-Pekkanen R, Muller N, Colby TV, Schuyler M, Cormier Y. HP Study Group. Clinical diagnosis of hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2003. 168:952–958.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download