Abstract
Neurodegenerative diseases are highly morbid and widespread in the nation with aged population. Since these are progressive and irreversible diseases, early detection and differentiation of the disease are important for possible therapeutic intervention. Alzheimer's disease and Parkinson's disease are the most frequent and costly devastating neurodegenerative diseases. Recent advances of molecular imaging, especially positron emission tomography (PET) technique, allows non-invasive evaluation of not only regional cerebral metabolism or perfusion, but also the change of neurotransmission and presence of abnormal protein such as beta amyloid. In Parkinsonism, dopamine transporter and vesicular monoamine transporter imaging are useful in the diagnosis and evaluation of the disease progression since these provide information about the integrity of presynaptic striatal dopaminergic neurons. In Alzheimer's disease, beta-amyloid imaging can assess the amyloid deposition. It improves early diagnosis and possibility of a presymptomatic diagnostic biomarker; improves understanding of the natural history of amyloid deposition; and has the capability to directly measure the effects of newly developed anti-amyloid therapies. Cholinergic and microglial imaging can be also useful in the early diagnosis of dementia and improves understanding of insights into pathophysiology of neurodegenerative diseases. Therefore, the ability of molecular imaging to identify and quantify cerebral pathology has significant implications for early detection, differential diagnosis, and therapeutic monitoring in neurodegenerative diseases.
Figures and Tables
Figure 3
[18F]FP-CIT PET images of normal healthy person and patients with early stage (H&Y stage I) and advanced stage (H&Y stage III) of Parkinson's disease. In early Parkinson's disease, bilateral putamina show asymmetrically decreased uptake. Advanced Parkinson's disease show significantly decreased uptake not only in bilateral putamina but also caudate nuclei.
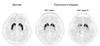
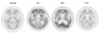
Figure 5
[11C]PIB PET images of normal healthy person and patients with Alzheimer's disease, Mild cognitive impairment, and frontotemporal dementia. In [11C]PIB PET images of normal person and patient with frontotemporal dementia, cerebral cortical uptake is lower than white matter. [11C]PIB PET image of Alzheimer's disease shows increased uptake in bilateral frontal and temporal cortex, and anterior striata. [11C]PIB PET image of mild cognitive impairment also shows increased uptake in bilateral posterior cingulate gyri, suggesting increased beta-amyloid burden.
References
1. Kaufman MJ, Madras BK. Severe depletion of cocaine recognition sites associated with the dopamine transporter in Parkinson's-diseased striatum. Synapse. 1991. 9:43–49.

2. Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973. 20:415–455.

3. Brooks DJ. Functional imaging in relation to parkinsonism syndromes. J Neurol Sci. 1993. 115:1–17.
4. Neumeyer JL, Wang S, Gao Y, Milius RA, Kula NS, Campbell A, Baldessarini RJ, Zea-Ponce Y, Baldwin Innis RB. N-α Fluoroalkyl analogs of (1R)-2β-carbomethoxy-3β-(4-iodophenyl)-tropane (β-CIT): Radiotracers for positron emission tomography and single photon emission computed tomography imaging of dopamine transporter. J Med Chem. 1994. 37:1558–1561.

5. Laruelle M, Baldwin RM, Malison RT, Zea-Ponce Y, Zoghbi SS, al-Tikriti MS. SPECT imaging of dopamine and serotonin transporters with [123I]beta-CIT: pharmacological characterization of brain uptake in nonhuman primates. Synapse. 1993. 13:295–309.

6. Haaparanta M, Bergman J, Laakso A, Hietala J, Solin O. [18F]CFT ([18F]WIN 35,428), a radioligand to study the dopamine transporter with PET: biodistribution in rats. Synapse. 1996. 23:321–327.

7. Huang WS, Lee MS, Lin JC, Chen CY, Yang YW, Lin SZ, Wey SP. Usefulness of brain 99mTc-TRODAT-1 SPET for the evaluation of Parkinsons disease. Eur J Nucl Med Mol Imaging. 2004. 31:155–161.

8. Kim HJ, Im JH, Yang SO, Moon DH, Ryu JS, Bong JK, Nam KP, Cheon JH, Lee MC, Lee hK. Imaging and quantitation of dopamine transporters with iodine-123-IPT in normal and Parkinson's disease subjects. J Nucl Med. 1997. 38:1703–1711.
9. Lundkvist C, Halldin C, Ginovart N, Swahn CG, Farde L. [18F] beta-CIT-FP is superior to [11C] beta-CIT-FP for quantitation of the dopamine transporter. Nucl Med Biol. 1997. 24:621–627.

10. Lee SJ, Oh SJ, Chi DY, Kang SH, Kil HS, Kim JS, Moon DH. One-step high-radiochemical-yield synthesis of [18F]FP-CIT using a protic solvent system. Nucl Med Biol. 2007. 34:345–351.

11. Kazumata K, Dhawan V, Chaly T, Antonini A, Margouleff C, Belakhlef A, Neumeyer J, Eidelberg D. Dopamine transporter imaging with fluorine-18-FPCIT and PET. J Nucl Med. 1998. 39:1521–1530.
12. Ma Y, Mentis DM, Chaly T, Spetsieris PG, Eidelberg D. Parametric mapping of [18F]FPCIT binding in early stage Parkinson's disease: a PET study. Synapse. 2002. 45:125–133.

13. Booij J, Tissingh G, Boer GJ, Speelman JD, Stoof JC, Janssen AG, Wolters EC, van Royen EA. [123I]FP-CIT SPECT shows a pronounced decline of striatal dopamine transporter labelling in early and advanced Parkinson's disease. J Neurol Neurosurg Psychiatry. 1997. 62:133–140.

14. Rinne JO, Ruottinen H, Bergman J, Haaparanta M, Sonninen P, Solin O. Usefulness of a dopamine transporter PET ligand [18F]beta-CFT in assessing disability in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1999. 67:737–741.

15. Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991. 114:2283–2301.

16. Pircker W, Asenbaum S, Hauk M, Kandlhofer S, Tauscher J, Willeit M, Neumeister A, Praschak-Rieder N, Angelberger O, Brcke T. Imaging serotonin and dopamine transporters with 123I-B-CIT SPECT: Binding kinetics and effects of normal aging. J Nucl Med. 2000. 41:36–44.
17. Mozley PD, Acton PD, Barraclough ED, Plssl K, Gur RC, Alavi A, Mathur A, Saffwe J, Kung HK. Effects of age on dopamine transporters in healthy humans. J Nucl Med. 1999. 40:1812–1817.
18. Lavalaye J, Booij J, Reneman L, Habraken JBA, van Royen EA. Effect of age and gender on dopamine transporter imaging with [123I]FP-CIT SPET in healthy volunteers. Eur J Nucl Med. 2000. 27:867–869.

19. Whone AL, Watts RL, Stoessl AJ, Davis M, Reske S, nahmias C, Lang AE, Rascol O, Ribeiro MJ, Remy P, Poewe WH, Hauser RA, Brooks DJ. Slower progression of Parkinson's disease with ropinirole versus levodopa: the REALPET study. Ann Neurol. 2003. 54:93–101.

20. Parkinson Study Group. Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA. 2002. 287:1653–1661.
21. The Parkinson Study Group. Levodopa and the progression of Parkinson's disease. N Engl J Med. 2004. 351:2498–2508.
22. Rajput A, Robinson CA, Rajput AH. Essential tremor course and disability: a clinicopathologic study of 20 cases. Neurology. 2004. 62:932–936.

23. Brooks DJ, Playford ED, Ibanez V, Sawle GV, Thompson PH, Findley LJ, Marsden CD. Isolated tremor and disruption of the nigrostriatal dopaminergic system: an 18F-dopa PET study. Neurology. 1992. 42:1554–1560.

24. Meara J, Bhowmick BK, Hobson P. Accuracy of diagnosis in patients with presumed Parkinson's disease. Age Ageing. 1999. 28:99–102.

25. Benamer HTS, Patterson J, Grosset DG. Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]FP-CIT SPECT imaging: the [123I]FP-CIT SPECT study group. Mov Disord. 2000. 15:503–510.
26. Chaudhuri KR, Buxton-Thomas M, Dhawan V, Peng R, Meilak C, Brooks DJ. Long duration asymmetrical postural tremor is likely to predict development of Parkinson's disease and not essential tremor: clinical follow up study of 13 cases. J Neurol Neurosurg Psychiatry. 2005. 76:115–117.

27. Varrone A, Marek KL, Jennings D, Innis RB, Seibyl JP. [1231I]-CIT SPECT imaging demonstrates reduced density of striatal dopamine transporters in Parkinson's disease and multiple system atrophy. Mov Disord. 2001. 16:1023–1032.

28. Antonini A, Benti R, De Notaris R, Tesei S, Zecchinelli A, Sacilotto G, Meucci N, Canesi M, Mariani C, Pezzoli G, Gerundini P. 123I-Ioflupane/SPECT binding to striatal dopamine transporter (DAT) uptake in patients with Parkinson's disease, multiple system atrophy, and progressive supranuclear palsy. Neurol Sci. 2003. 24:149–150.

29. Plotkin M, Amthauer H, Klaffke S, Khn A, Ldemann L, Arnold G, Wernecke KD, Kupsch A, Felix R, Venz S. Combined [123]IFPCIT and [123]I-IBZM SPECT for the diagnosis of parkinsonian syndromes: study on 72 patients. J Neural Transm. 2005. 112:677–692.

30. Scherfler C, Seppi K, Donnemiller E, Goebel G, Brenneis C, Virgolini I, Wenning GK, Poewe W. Voxel-wise analysis of [123I]-CIT SPECT differentiates the Parkinson variant of multiple system atrophy from idiopathic Parkinson's disease. Brain. 2005. 128:1605–1612.

31. Seppi K, Scherfler C, Donnemiller E, Virgolini I, Schocke MF, Goebel G, Mair KJ, Boesch S, Brenneis C, Wenning GK, Poewe W. Topography of dopamine transporter availability in progressive supranuclear palsy: a voxel wise [123I]CIT SPECT analysis. Arch Neurol. 2006. 63:1154–1160.

33. Zijlmans JCM, Daniel SE, Hughes AJ, Re've'sz T, Lees AJ. Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Mov Disord. 2004. 19:630–640.

34. Gerschlager W, Bencsits G, Pirker W, Bloem BR, Asenbaum S, Prayer D, Zijlmans JC, Hoffmann M, Brcke T. [123I]-CIT distinguishes vascular parkinsonism from Parkinson's disease. Mov Disord. 2002. 17:518–523.

35. Lorberboym M, Djaldetti R, Melamed E, Sadeh M, Lampl Y. [123I]FP-CIT SPECT Imaging of dopamine transporters in patients with cerebrovascular disease and clinical diagnosis of vascular parkinsonism. J Nucl Med. 2004. 45:1688–1693.
36. Caligiuri MP, Bracha HS, Lohr JB. Asymmetry of neuroleptic induced rigidity: development of quantitative methods and clinical correlates. Psychiatry Res. 1989. 30:275–284.

37. Lavalaye J, Linszen DH, Booij J, Dingemans PM, Reneman L, Habraken JB, Gersons BP, van Royen EA. Dopamine transporter density in young patients with schizophrenia assessed with [123]FP-CIT SPECT. Schizophr Res. 2001. 47:59–67.

38. McKhann G, Drachman D, Folstein MF, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer s disease. Neurology. 1984. 34:939–944.

39. Merdes AR, Hansen LA, Jeste DV, Galasko D, Hofstetter CR, Ho GJ, Thal LJ, Corey-Bloom J. Influenceof Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology. 2003. 60:1586–1590.

40. Eerola J, Tienari PJ, Kaakkola S, Nikkinen P, Launes J. How useful is [123I]-CIT SPECT in clinical practice? J Neurol Neurosurg Psychiatry. 2005. 76:1211–1216.
41. Costa DC, Walker Z, Walker RW, Fontes FR. Dementia with Lewy bodies versus Alzheimer's disease: role of dopamine transporter imaging. Mov Disord. 2003. 18:S7. 34–38.

42. Ponsen MM, Stoffers D, Booij J, van Eck-Smit BL, Wolters ECh, Berendse HW. Idiopathic hyposmia as a preclinical sign of Parkinson's disease. Ann Neurol. 2004. 56:173–181.

43. Zommer U, Hummel T, Cormann K, Mueller A, Frasnelli J, Kropp J, Reichmann H. Detection of presymptomatic Parkinson's disease: combining smell tests, transcranial sonography, and SPECT. Mov Disord. 2004. 19:1196–1202.

44. Iranzo A, Santamara J, Rye DB, Valldeoriola F, Mart MJ, Muoz E, Vilaseca I, Tolosa E. Characteristics of idiopathic REM sleep behavior disorder and that associated with MSA and PD. Neurology. 2005. 65:247–252.

46. Perl DP. Neuropathology of Alzheimer's disease and related disorders. Neurol Clin. 2000. 18:847–864.

47. Lovestone S, Reynolds CH. The phosphorylation of tau: a critical stage in neurodevelopment and neurodegenerative processes. Neuroscience. 1997. 78:309–324.
48. Cappai R, White AR. Amyloid beta. Int J Biochem Cell Biol. 1999. 31:885–889.
49. Harper JD, Lansbury PT Jr. Models of amyloid seeding in Alzheimer's disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem. 1997. 66:385–407.

50. Seubert P, Vigo-Pelfrey C, Esch F, Lee M, dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C. Isolation and quanti-fication of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992. 359:325–327.

51. Dickson TC, Vickers JC. The morphological phenotype of betaamyloid plaques and associated neuritic changes in Alzheimer's disease. Neuroscience. 2001. 105:99–107.

52. Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE, McDonald WC, Braak H, Petersen RC. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003. 62:1087–1095.

53. Pearson HA, Peers C. Physiological roles for amyloid beta peptides. J Physiol. 2006. 575:5–10.
54. Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003. 46:2740–2754.

55. Zhang W, Oya S, Kung MP, Hou C, Maier DL, Kung HF. F-18 Polyethyleneglycol stilbenes as PET imaging agents targeting Abeta aggregates in the brain. Nucl Med Biol. 2005. 32:799–809.

56. Luurtsema G, Schuit RC, Takkenkamp K, Lubberink M, Hendrikse NH, Windhorst AD, Molthoff CF, Tolboom N, van Berckel BN, Lammertsma AA. Peripheral metabolism of [(18)F]FDDNP and cerebral uptake of its labelled metabolites. Nucl Med Biol. 2008. 35:869–874.

57. Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrm M, Savitcheva I, Huang GF, Estrada S, Ausn B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Lngstrm B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004. 55:306–319.

58. Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, Holt DP, Meltzer CC, Dekosky ST, Mathis CA. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005. 25:1528–1547.

59. Verhoeff NP, Wilson AA, Takeshita S, Trop L, Hussey D, Singh K, Kung HF, Kung MP, Houle S. In-vivo imaging of Alzheimer disease betaamyloicwith [11C]SB-13 PET. Am J Geriatr Psychiatry. 2004. 12:584–595.
60. Small GW, Kepe V, Ercoli LM, Siddarth P, Bookheimer SY, Miller KJ, Lavretsky H, Burggren AC, Cole GM, Vinters HV, Thompson PM, Huang SC, Satyamurthy N, Phelps ME, Barrio JR. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med. 2006. 355:2652–2663.

61. Rabinovici GD, Furst AJ, O'Neil JP, Racine CA, Mormino EC, Baker SL, Chetty S, Patel P, Pagliaro TA, Klunk WE, Mathis CA, Rosen HJ, Miller BL, Jagust WJ. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2007. 68:1205–1212.

62. Forsberg A, Engler H, Almkvist O, Blomquist G, Hagman G, Wall A, Ringheim A, Lngstrm B, Nordberg A. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging. 2008. 29:1456–1465.

63. Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko sK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, Dekosky ST, halligan EM, Klunk WE. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008. 65:1509–1517.

64. Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C] PIB in a nondemented population. Potentional antecendent marker of Alzheimer's disease. Neurology. 2006. 67:446–452.

65. Price JL, Morris JC. Tangles and plaques in nondemented aging and preclinical Alzheimer's disease. Ann Neurol. 1999. 45:358–368.

66. Ritchie CW, Bush AI, Mackinnon A, Macfarlane S, mastwyk M, Macgregor L, Kiers L, Cherny R, Li QX, Tammer A, Carrington D, Mavros C, Volitakis I, Xilinas m, Ames D, Davis S, Beyreuther K, Tanzi RE, Masters CL. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch Neurol. 2003. 60:1685–1691.

67. Schenk D, Hagen M, Seubert P. Current progress in betaamyloid immunotherapy. Curr Opin Immunol. 2004. 16:599–606.

68. Blokland A. Acetylcholine: A Neurotransmitter for Learning and Memory? Brain Res Rev. 1996. 21:285–300.

69. Mesulam MM, Mufson EJ, Wainer BH, Levy AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (CH1-CH-6). Neurosci. 1983. 10:1185–1201.
70. De Lacalle S, Lim C, Sobreviela T, Mufson E, Hersh L, Saper C. Cholinergic innervation in the human hippocampal formation including the entorhinal cortex. J Comp Neurol. 1994. 345:321–344.

71. Lippa CF, Smith TW, Perry E. Dementia with Lewy bodies: choline acetyltransferase parallels nucleus basalis pathology. J Neural Transm. 1999. 106(5-6):525–535.

72. Zea-Ponce Y, Mavel S, Assaad T, Kruse SE, Parsons SM, Emond P, Chalon S, Giboureau N, Kassiou M, Guilloteau D. Synthesis and in vitro evaluation of new benzovesamicol analogues as potential imaging probes for the vesicular acetylcholine transporter. Bioorg MedChem. 2005. 13:745–753.

73. Carpinelli A, Magni F, Cattaneo A, Matarrese M, Turolla E, Todde S, Bosso N, Kienle MG, Fazio F. Improved synthesis and radiolabeling of [11C]MP4A, a suitable ligand for the investigation of the cholinergic system using PET. Appl Radiat Isot. 2006. 2:182–186.
74. Snyder SE, Tluczek L, Jewett DM, Nguyen TB, Kuhl DE, Kilbourn MR. Synthesis of 1-[11C]methylpiperidin-4-yl propionate ([11C]PMP) for in vivo measurements of acetylcholinesterase activity. Nucl Med Biol. 1998. 25:751–754.

75. Norbury R, Travis MJ, Erlandsson K, Waddington W, Owens J, Ell PJ, Murphy DG. SPET imaging of central muscarinic receptors with (R,R)[123I]-I-QNB: methodological consi-derations. Nucl Med Biol. 2004. 31:583–590.

76. Lee KS, He X, Jones DW, Coppola R, Gorey JG, Knable MB, deCosta BR, Rice KC, Weinberger DR. An Improved Method for Rapid and Efficient Radioiodination of Iodine-123-IQNB. J Nucl Med. 1996. 37:2021–2024.
77. Iyo M, Namba H, Fukushi K, Shinotoh H, Nagatsuka S, Suhara T, Sudo Y, Suzuki K, Irie T. Measurement of acetylcholinesterase by positron emission tomography in the brains of healthy controls and patients with Alzheimer's disease. Lancet. 1997. 349:1805–1809.

78. Kuhl DE, Koeppe RA, Minoshima S, Snyder SE, Ficaro EP, Foster NL, Frey KA, Kibourn MR. In vivo mapping of cerebral acetylcholinesterase activity in aging and Alzheimer's disease [see comments]. Neurology. 1999. 52:691–699.

79. Shinotoh H, Namba H, Fukushi K, Nagatsuka S, Tanaka N, Aotsuka A, Tanada S, Irie T. Brain acetylcholinesterase activity in Alzheimer disease measured by positron emission tomography. Alzheimer Dis Assoc Disord. 2000. 14:S114–S118.

80. Bohnen NI, Kaufer DI, Hendrickson R, Ivanco LS, Lopresti B, Davis JG, Constantine G, Mathis CA, Moore RY, DeKosky ST. Cognitive correlates of alterations in acetylcholinesterase in Alzheimer' disease. Neurosci Lett. 2005. 380:127–132.

81. Bohnen NI, Kaufer DI, Ivanco LS, Lopresti B, Koeppe RA, Davis JG, Mathis CA, Moore RY, Dekosky ST. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch Neurol. 2003. 60:1745–1748.

82. Herholz K, Weisenbach S, Kalbe E, Diederich NJ, Heiss WD. Cerebral acetylcholine esterase activity in mild cognitive impairment. Neuroreport. 2005. 16:1431–1434.

83. Nordberg A, Lundqvist H, Hartvig P, Lilja A, Langstrom B. Kinetic analysis of regional (S)(-)11C-nicotine binding in normal and Alzheimer brains-in vivo assessment using positron emission tomography. Alzheimer Dis Assoc Disord. 1995. 9:21–27.

84. Kuhl DE, Minoshima S, Frey KA, Foster NL, Kilbourn MR, Koeppe RA. Limited donepezil inhibition of acetylcholinesterase measured with positron emission tomography in living Alzheimer cerebral cortex. Ann Neurol. 2000. 48:391–395.

85. Bohnen NI, Kaufer DI, HendricksonR R, Ivanco LS, Lopresti BJ, Koeppe RA, Meltzer CC, Constantine G, Davis JG, Mathis CA, Dekosky ST, Moore RY. Degree of inhibition of cortical acetylcholinesterase activity and cognitive effects by donepezil treatment in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2005. 76:315–319.

86. Kaasinen V, Någren K, Jävenpää T, Roivainen A, Yu M, Oikonen V, Kurki T, Rinne JO. Regional effects of donepezil and rivastigmine on cortical acetylcholinesterase activity in Alzheimer's disease. J Clin Psychopharmacol. 2002. 22:615–620.

87. Nordberg A, Lundqvist H, Hartvig P, Andersson J, Johansson M, Hellstrom-Lindahi E, Lngstrm B. Imaging of nicotinic and muscarinic receptors in Alzheimer's disease: effect of tacrine treatment. Dement Geriatr Cogn Disord. 1997. 8:78–84.

88. Minghetti L, Levi G. Microglia as effector cells in brain damage and repair: focus on prostanoids and nitric oxide. Prog Neurobiol. 1998. 54:99–125.

89. Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev. 1995. 20:269–287.

90. Chan A, Seguin R, Magnus T, Papadimitriou C, Toyka KV, Antel JP, Gold R. Phagocytosis of apoptotic inflammatory cells by microglia and its therapeutic implications: termination of CNS autoimmune inflammation and modulation by interferonbeta. Glia. 2003. 43:231–242.

91. Gonzalez-Scarano F, Baltuch G. Microglia as mediators of nflammatory and degenerative diseases. Annu Rev Neurosci. 1999. 22:219–240.
92. McGeer PL, Itagaki S, Tago H, McGeer EG. Occurrence of HLADR reactive microglia in Alzheimer's disease. Ann N Y Acad Sci. 1988. 540:319–323.

93. McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson s and Alzheimer's disease brains. Neurology. 1988. 38:1285–1291.

94. Chao CC, Hu S, Peterson PK. Modulation of human microglial cell superoxide production by cytokines. J Leukoc Biol. 1995. 58:65–70.

95. Colton CA, Keri JE, Chen WT, Monsky WL. Protease production by cultured microglia: substrate gel analysis and immobilized matrix degradation. J Neurosci Res. 1993. 35:297–304.

96. Giulian D, Vaca K, Noonan CA. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990. 250:1593–1596.

97. Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, Seubert P, Games D, Kirby L, Schenk D. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2006. 64:129–131.

98. Braestrup C, Albrechtsen R, Squires RF. High densities of benzodiazepine receptors in human cortical areas. Nature. 1977. 269:702–704.

99. Casellas P, Galiegue S, Basile AS. Peripheral benzodiazepine receptors and mitochondrial function. Neurochem Int. 2002. 40:475–486.

100. McEnery MW, Snowman AM, Trifiletti RR, Snyder SH. Isolation of the mitochondrial benzodiazepine receptor: association with the voltagedependent anion channel and the adenine nucleotide carrier. Proc Natl Acad Sci USA. 1992. 89:3170–3174.

101. Shah F, Hume SP, Pike VW, Ashworth S, McDermott J. Synthesis of the enantiomers of [N-methyl-11C]PK 11195 and comparison of their behaviours as radioligands for PK binding sites in rats. Nucl Med Biol. 1994. 21:573–581.

102. Yu W, Wang E, Voll RJ, Millerb AH, Goodman MM. Synthesis, fluorine-18 radiolabeling, and in vitro characterization of 1-iodophenyl-N-methyl-N-fluoroalkyl-3-isoquinoline carboxamide derivatives as potential PET radioligands for imaging peripheral benzodiazepine receptor. Bioor Med Chem. 2008. 16:6145–6155.

103. Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, Jones T, Banati RB. In-vivo measurement of activated microglia in dementia. Lancet. 2001. 358:461–467.

104. Groom GN, Junck L, Foster NL, Frey KA, Kuhl DE. PET of Peripheral benzodiazepine binding sites in the microgliosis of Alzheimer's Disease. J Nuci Med. 1995. 36:2207–2210.
105. Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiol Dis. 2006. 21:404–412.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download