Abstract
Molecular imaging is a bioimaging that can detect biochemically and genetically relevant events in molecular level in cells and tissues via quantitative imaging signal. Molecular imaging provides potential advantages to examine early diagnosis of specific diseases, to screen new candidates of a drug, to monitor therapeutic effects in real time, and to communicate with both diagnosis and therapeutics. These diverse advantages of molecular imaging can be allowed by development of nanoplatform technology. The nanoplatform-based probes for molecular imaging is widely investigated to grant multimodal molecular imaging and drug delivery together with medical imagings, which includes the issues of biocompatibility, targeting moiety, protease-specific peptide substrate, quenching/dequenching system etc. In this paper, nanoplatform-based probes are reviewed in aspects of cancer targeting for diagnosis and therapy and multimodal molecular imaging with inorganic/organic hybrid nanoparticles.
Figures and Tables
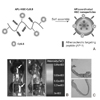 | Figure 1Hydrophobically modified chitosan (HGC) nanoparticles targeting for atherosclerosis via homing peptide, (A) Schematic picture of atherosclerotic homing peptide conjugated HGC, (B) NIR fluorescence imaging that HGCs were localized on atherosclerotic regions in vivo, (C) histological morphology of atherosclerotic region of aorta vessel. Modified with permission from Ref. 5. Copyright ©, 2008 Elsevier C.V. |
 | Figure 2Nanostructure of polymersome, (A) schematic nanostructure of polymersome and dye loading within hydrophobic bilayer, (B) NIR fluorescence imaging in vivo via polymersome probe. Modified with permission from ref. 6. Cpolyright 2005 PNAS. |
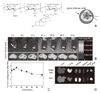 | Figure 3HGC nanoparticle for cancer imaging, (A) schematic picture of Cy 5.5 labeled HGC, (B) NIR fluorescence imaging in vivo for tumor targeting using Cy 5.5 labeled HGC nanoparticles, (C) Quantitative analysis of fluorescence signal for tumor to background signal ratio, (D) biodistribution in organs ex vivo. Modifiedwith permission from ref. 7. Copyright © 2008 Elsevier B.V. |
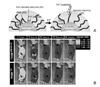 | Figure 4pH sensitive micelle with cell penetrating peptide TAT, (A) schematic picture to show the structural change of micelle at pH variance. TAT moieties are faced outward at weak acid of tumor site. (B) NIR fluorescence imaging in vivo of tumor targeting via pH sensitive micelle with TAT moieties. Modified with permission from ref. 12. Copyright © 2008 Elsevier B.V. |
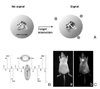 | Figure 5Protease-specific nanoparticle probe, (A) Schematic picture of poly (L-Lysine) nanoparticles, (B) Schematric picture of selective degradation of polymer main backbone by protease, (C) NIR fluorescence bioimaging that was emitted by the cleavage of lysine substrates. Modified with permission from ref. 21. Copyright 1999, Nature Publishing Group. |
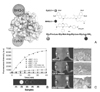 | Figure 6MMP specific peptide probes (A) Schematic picture of cleavage mechanism between MMP and MMP specific peptide substrate that was quenched by Cy5.5/BHQ system, (B) Sensitive fluorescence emission by MMP cleavage dependence in vitro characterization, (C) NIR fluorescence bioimaging that was activated by MMP in tumor site via MMP activatable probe. Modified with permission from 19. Copyright 2008, American Chemical Society. |
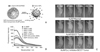 | Figure 7Inorganic MMP activatable gold nanoprobe (A) schematic picture of gold nanoprobe that is activated by cleavage of MMP specific peptide substrate, (B) in vitro characterization of MMP dependence of MMP activatable gold nanoparticles, (C) NIR fluorescence imaging, Fluorescence intensity is emitted in only tumor site by MMP cleavage of peptide substrate that was conjugated on gold nanoparticle. Quenched fluorescence probe by gold nanoparticles was emitted as Cy 5.5 was departed from gold nanoparticles. Modified with permission from ref. 18. Copyright © 2008 WILEY-VCH Verlag GmbH & Co. |
 | Figure 8Multifunctional quantum dots with dual imaging modality, (A) schematic picture of multifunctional quantum dot nanoparticles with multimodality. Multifunctional nanocomplex;Paramagnetic lipid for MRI and quantum doe for fluorescence, RGD peptide for targeting, PEG for biocompatibility, (B) MRI image administered with multifunctional quantum dots, (C) Fluorescence bioimaging. Modified with permission from ref. 29. Copyright © 2008 Springer. |
Table 1
Target protease and peptide substrates for specific cancers and diseases

*This table was modified with permission (33).
References
2. Park K, Kim JH, Nam YS, Lee S, Nam HY, Kim K, Park JH, Kim IS, Choi K, Kim SY, Kwon IC. Effect of polymer molecular weight on the tumor targeting characteristics of self-assembled glycol chitosan nanoparticles. J Control Release. 2007. 122:305–314.

3. Thapa N, Hong HY, Sangeetha P, Kim IS, Yoo J, Rhee K, Oh GT, Kwon IC, Lee BH. Identification of a peptide ligand recognizing dysfunctional endothelial cells for targeting atherosclerosis. J Control Release. 2008. 131:27–33.

4. Hong HY, Lee HY, Kwak W, Yoo J, Na MH, So IS, Kwon TH, Park HS, Huh S, Oh GT, Kwon IC, Kim IS, Lee BH. Phage Display Selection of Peptides that Home to Atherosclerotic Plaques: IL-4 Receptor as a Candidate Target in Atherosclerosis. J Cell Mol Med. 2007.

5. Park K, Hong HY, Moon HJ, Lee BH, Kim IS, Kwon IC, Rhee K. A new atherosclerotic lesion probe based on hydrophobically modified chitosan nanoparticles functionalized by the atherosclerotic plaque targeted peptides. J Control Release. 2008. 128:217–223.

6. Ghoroghchian PP, Frail PR, Susumu K, Blessington D, Brannan AK, Bates FS, Chance B, Hammer DA, Therien MJ. Near-infrared-emissive polymersomes: self-assembled soft matter for in vivo optical imaging. Proc Natl Acad Sci U S A. 2005. 102:2922–2927.

7. Hwang HY, Kim IS, Kwon IC, Kim YH. Tumor targetability and antitumor effect of docetaxel-loaded hydrophobically modified glycol chitosan nanoparticles. J Control Release. 2008. 128:23–31.

8. Cho YW, Park SA, Han TH, Son DH, Park JS, Oh SJ, Moon DH, Cho KJ, Ahn CH, Byun Y, Kim IS, Kwon IC, Kim SY. In vivo tumor targeting and radionuclide imaging with self-assembled nanoparticles: mechanisms, key factors, and their implications. Biomaterials. 2007. 28:1236–1247.

9. Min KH, Park K, Kim YS, Bae SM, Lee S, Jo HG, Park RW, Kim IS, Jeong SY, Kim K, Kwon IC. Hydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapy. J Control Release. 2008. 127:208–218.

10. Kim JH, Kim YS, Park K, Lee S, Nam HY, Min KH, Jo HG, Park JH, Choi K, Jeong SY, Park RW, Kim IS, Kim K, Kwon IC. Antitumor efficacy of cisplatin-loaded glycol chitosan nanopar-ticles in tumor-bearing mice. J Control Release. 2008. 127:41–49.

11. Yin H, Lee ES, Kim D, Lee KH, Oh KT, Bae YH. Physicochemical characteristics of pH-sensitive poly (L-histidine)-b-poly (ethylene glycol)/poly (L-lactide)-b-poly (ethylene glycol) mixed micelles. J Control Release. 2008. 126:130–138.

12. Lee ES, Gao Z, Kim D, Park K, Kwon IC, Bae YH. Super pH-sensitive multifunctional polymeric micelle for tumor pH (e) specific TAT exposure and multidrug resistance. J Control Release. 2008. 129:228–236.

13. Pham W, Pantazopoulos P, Moore A. Imaging Farnesyl Protein Transferase Using a Topologically Activated Probe. J Am Chem Sco. 2006. 128:11736–11737.

14. Ntziachristos V, Schellenberger EA, Ripoll J, Yessayan D, Graves E, Bogdanov A, Josephson L, Weissleder R. Visualization of antitumor treatment by means of fluorescence molecular tomography with an annexin V-Cy5.5 conjugate. Proc Natl Acad Sci U S A. 2004. 101:12294–12299.

15. Jaffer FA, Kim DE, Quinti L, Tung CH, Aikawa E, Pande AN, Kohler RH, Shi GP, Libby P, Weissleder R. Optical Visualization of Cathepsin K Activity in Atherosclerosis With a Novel, Protease-Activatable Fluorescence Sensor. Circulation. 2007. 115:2292–2298.

16. Fischer R, Bachle D, Fotin-Mleczek M, Jung G, Kalbacher H, Brock R. A targeted protease substrate for a quantitative determination of protease activities in the endolysosomal pathway. Chembiochem. 2006. 7:1428–1434.

17. Bremer C, Tung CH, Weissleder R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat Med. 2001. 7:743–748.

18. Lee S, Cha EJ, Park K, Lee SY, Hong JK, Sun IC, Kim SY, Choi K, Kwon IC, Kim K, Ahn CH. A near-infrared-fluorescence-quenched gold-nanoparticle imaging probe for in vivo drug screening and protease activity determination. Angew Chem Int Ed Engl. 2008. 47:2804–2807.

19. Lee S, Park K, Lee SY, Ryu JH, Park JW, Ahn HJ, Kwon IC, Youn IC, Kim K, Choi K. Dark quenched matrix metalloproteinase fluorogenic probe for imaging osteoarthritis development in vivo. Bioconjug Chem. 2008. 19:1743–1747.

20. Lee S, Park K, Kim K, Choi K, Kwon IC. Activatable imaging probes with amplified fluorescent signals. Chem Commun (Camb). 2008. 4250–4260.

21. Weissleder R, Tung CH, Mahmood U, Bogdanov A Jr. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999. 17:375–378.

22. Wunder A, Tung CH, Muller-Ladner U, Weissleder R, Mahmood U. In vivo imaging of protease activity in arthritis: a novel approach for monitoring treatment response. Arthritis Rheum. 2004. 50:2459–2465.

23. Law B, Weissleder R, Tung C-H. Mechanism-Based Fluorescent Reporter for Protein Kinase A Detection. Chem Bio Chem. 2005. 6:1361–1367.

24. Rabin O, Manuel Perez J, Grimm J, Wojtkiewicz G, Weissleder R. An X-ray computed tomography imaging agent based on long-circulating bismuth sulphide nanoparticles. Nat Mater. 2006. 5:118–122.

25. Perez JM, Josephson L, O'Loughlin T, Högemann D, Weissleder R. Magnetic relaxation switches capable of sensing molecular interactions. Nat Biotechnol. 2002. 20:816–820.

26. von zur Muhlen C, von Elverfeldt D, Moeller JA, Choudhury RP, Paul D, Hagemeyer CE, Olschewski M, Becker A, Neudorfer I, Bassler N, Schwarz M, Bode C, Peter K. Magnetic resonance imaging contrast agent targeted toward activated platelets allows in vivo detection of thrombosis and monitoring of thrombolysis. Circulation. 2008. 118:258–267.

27. Gao X, Yang L, Petros JA, Marshall FF, Simons JW, Nie S. In vivo molecular and cellular imaging with quantum dots. Curr Opin Biotechnol. 2005. 16:63–72.

28. Kamaly N, Kalber T, Ahmad A, Oliver MH, So PW, Herlihy AH, Bell JD, Jorgensen MR, Miller AD. Bimodal paramagnetic and fluorescent liposomes for cellular and tumor magnetic resonance imaging. Bioconjug Chem. 2008. 19:118–129.

29. Mulder WJ, Castermans K, van Beijnum JR, Oude Egbrink MG, Chin PT, Fayad ZA, Lowik CW, Kaijzel EL, Que I, Storm G, Strijkers GJ, Griffioen AW, Nicolay K. Molecular imaging of tumor angiogenesis using alphavbeta3-integrin targeted multimodal quantum dots. Angiogenesis. 2008.
30. Gerstl F, Windischberger C, Mitterhauser M, Wadsak W, Holik A, Kletter K, Moser E, Kasper S, Lanzenberger R. Multimodal imaging of human early visual cortex by combining functional and molecular measurements with fMRI and PET. Neuroimage. 2008. 41:204–211.

31. Choi JH, Nguyen FT, Barone PW, Heller DA, Moll AE, Patel D, Boppart SA, Strano MS. Multimodal biomedical imaging with asymmetric single-walled carbon nanotube/iron oxide nanoparticle complexes. Nano Lett. 2007. 7:861–867.

32. Cheon J, Lee JH. Synergistically Integrated Nanoparticles as Multimodal Probes for Nanobiotechnology. Acc Chem Res. 2008.

33. Nam HY, Park JH, Kwon IC. Polymer for Bioimaging. Polymer Sci Technol. 2008. 19:130–137.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download