Abstract
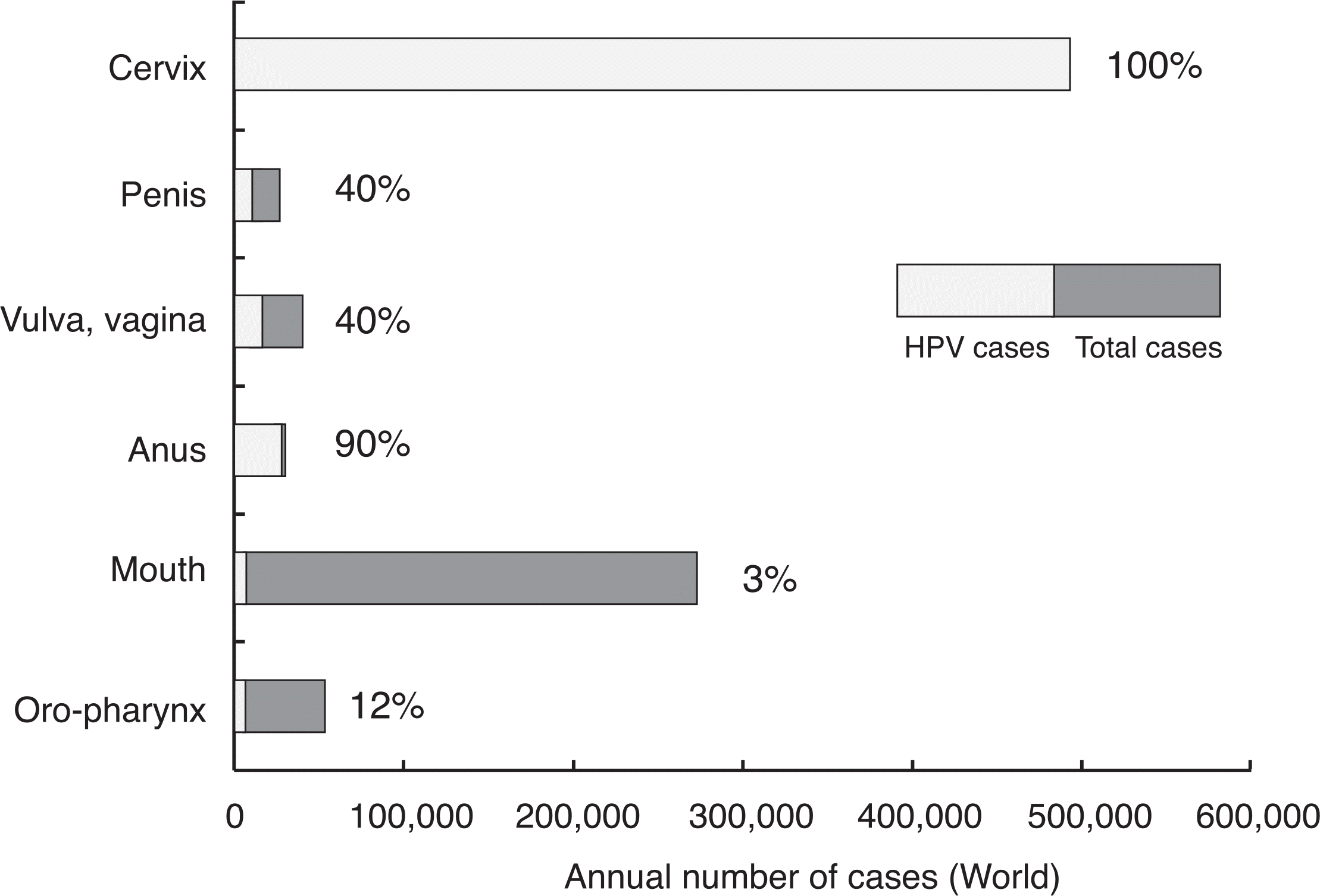
Cervical cancer is the second most common cancer affecting women worldwide. Cervical cancer is caused by persistent infection with high-risk types of human papillomavirus (HPV). The most common oncogenic HPV genotypes are 16 and 18, causing approximately 70% of all cervical cancers. Recently, two HPV vaccines, quadrivalent (HPV 6, 11, 16, 18) and bivalent (HPV 16, 18) vaccines, have been licensed and are now marketed in Korea. HPV vaccines are prepared from virus-like particles (VLPs) produced by recombinant technology. Clinical trials have confirmed that both vaccines have high efficiency against persistent infection of HPV 16 or 18 and moderate to severe precancerous lesions. In women who have no evidence of past or current infection with the HPV genotypes in the vaccine, both vaccines show > 90% protection against persistent HPV infection for up to 5 years after vaccination. In addition, vaccine efficacy against precancerous lesions associated with HPV 16/18 was reported to be 100%. Although most clinical trials to date have investigated the effectiveness of HPV vaccines in young females, elderly females and males may also be candidates for HPV vaccines. Since HPV vaccines are prophylactic, the largest impact of vaccination is expected to result from high coverage of young adolescents before exposure to HPV. Cervical cancer screening will still be required, even after HPV vaccines are introduced, although the screening program may need to be adapted to achieve cost-effective reductions in the burden of cervical cancer prevention strategies.
References
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.

2. Chung HH, Jang MJ, Jung KW, Won YJ, Shin HR, Kim JW Lee HP. Cervical cancer incidence and survival in Korea: 1993–2002. Int J Gynecol Cancer. 2006; 16:1833–1838.

3. Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, Markowitz LE. Prevalence of HPV infection among females in the United States. Jama. 2007; 297:813–819.

4. Koshiol J, Lindsay L, Pimenta JM, Poole C, Jenkins D, Smith JS. Persistent human papillomavirus infection and cervical neoplasia: a systematic review and metaanalysis. Am J Epidemiol. 2008; 168:123–137.

5. Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a metaanalysis. Br J Cancer. 2003; 88:63–73.

6. Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006; 24(Suppl 3):S3/11–25.

7. Derkay CS, Wiatrak B. Recurrent respiratory papillomatosis: a review. Laryngoscope. 2008; 118:1236–1247.

9. Carter JJ, Koutsky LA, Hughes JP, Lee SK, Kuypers J, Kiviat N, Galloway DA. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000; 181:1911–1919.

10. Hagensee ME, Yaegashi N, Galloway DA. Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J Virol. 1993; 67:315–322.

11. Mao C, Koutsky LA, Ault KA, Wheeler CM, Brown DR, Wiley DJ, Alvarez FB, Bautista OM, Jansen KU, Barr E. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006; 107:18–27.
12. Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, Wheeler CM, Koutsky LA, Malm C, Lehtinen M, Skjeldestad FE, Olsson SE, Steinwall M, Brown DR, Kurman RJ, Ronnett BM, Stoler MH, Ferenczy A, Harper DM, Tamms GM, Yu J, Lupinacci L, Railkar R, Taddeo FJ, Jansen KU, Esser MT, Sings HL, Saah AJ, Barr E. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005; 6:271–278.

13. Garlard SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, Tang GW, Ferris DG, Steben M, Bryan J, Taddeo FJ, Railkar R, Esser MT, Sings HL, Nelson M, Boslego J, Sattler C, Barr E, Koutsky LA. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007; 356:1928–1943.

14. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007; 356:1915–1927.
15. Ault KA. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007; 369:1861–1868.

16. Munoz N, Manalastas R Jr., Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, Clavel C, Luna J, Myers E, Hood S, Bautista O, Bryan J, Taddeo FJ, Esser MT, Vuocolo S, Haupt RM, Barr E, Saah A. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet. 2009; 373:1949–1957.
17. Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, Olsson SE, Hoye J, Steinwall M, Riis-Johannessen G, Andersson-Ellstrom A, Elfgren K, Krogh G, Lehtinen M, Malm C, Tammns GM, Giacoletti K, Lupinacci L, Railkar R, Taddeo FJ, Bryan J, Esser MT, Sings HL, Saah AJ, Barr E. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006; 95:1459–1466.

18. Olsson SE, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, Iversen OE, Hoye J, Stienwall M, Riis-Johannessen G, Andersson-Ellstrom A, Elfgren K, von Krogh G, Lehtinen M, Paavonen J, Tamms GM, Giacoletti K, Lupinacci L, Esser MT, Vuocolo SC, Saah AJ, Barr E. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine. 2007; 25:4931–4939.

19. Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, Hedrick J, Jaisamram U, Limson G, Garland S, Szarewski A, Romanowski B, Aoki FY, Schwarz TF, Poppe WA, Bosch FX, Jenkins D, Hardt K, Jahaf T, Descamps D, Struyf F, Lehtinen M, Dubin G, Greenacre M. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009; 374:301–314.

20. Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, Jenkins D, Schuind A, Costa Clemens SA, Dubin G. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006; 367:1247–1255.

21. Smith JF, Brownlow M, Brown M, Kowalski R, Esser MT, Ruiz W, Barr E, Brown DR, Bryan JT. Antibodies from women immunized with Gardasil cross-neutralize HPV 45 pseu-dovirions. Hum Vaccin. 2007; 3:109–115.
22. Brown DR, Kjaer Sk, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, Tay EH, Garcia P, Ault KA, Garland SM, Leodolter S, Olsson SE, Tang GW, Ferris DG, Paavonen J, Steben M, Bosch FX, Dillner J, Joura EA, Kurman RJ, Majewski S, Munoz N, Myers ER, Villa LL, Taddeo FJ, Roberts C, Tadesse A, Bryan J, Lupinacci LC, Giacoletti KE, Sings HL, James M, Hesley TM, Barr E. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis. 2009; 199:926–935.

23. Pataja T, Keranen H, Karppa T, Kawa A, Lantela S, Siitari-Mattila M, Levanen H, Tocklin T, Godeaux O, Lehtinen M, Dubin G. Immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in healthy boys aged 10–18 years. J Adolesc Health. 2009; 44:33–40.
Table 1.
Characteristics of the two HPV vaccines
Table 2.
Efficacy of the quadrivalent HPV vaccine among women who received three doses of vaccine according to protocol and had no evidence of past or current infection with the vaccine-related HPV genotypes.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download