Abstract
Because the 'Tsunami of type 2 diabetes' is presently rolling on a global scale, owing to the ever-increasing prevalence of obesity, increasing physical inactivity, and aging populations worldwide, the economic burden of diabetes caused by increased health resource use and lost productivity increase rapidly. So prevention in general population and good glycemic controls become even more important with earlier diagnosis and more aggressive cardiovascular prevention and treatment. Diabetes requires continuing medical care and patient self-management education to prevent acute complications and to reduce the risk of long-term complications. Diabetes care is very complex and requires that many issues, beyond glycemic control, be solved by the active governmental policy. Lifestyle modifications are the cornerstones of management of type 2 diabetes. The progressive nature of type 2 diabetes requires use of one or more oral agents and eventually insulin, along with lifestyle modification and intensification. Rapid achievement of the target goals often prompts providers to consider combination therapy to target different pathogenic mechanisms and manage both fasting and postprandial blood glucose levels. Maintenance of glycemic control over the lifespan of a patient with diabetes is overwhelmingly likely to require combination therapy with oral diabetes medications. Ultimately, because of the progressive nature of the disease and the progressive decline in pancreatic beta-cell function, insulin therapy is almost always obligatory to achieve optimal glycemic goals.
Figures and Tables
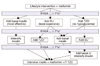 | Figure 1Algorithm for the metabolic management of type 2 diabetes mellitus (24). |
References
1. Korean Diabetes Association. Health Insurance Review & Assessment Service. Report of Task Force Team for Diabetes Basic Statistics Study Group: Diabetes in Korea. 2007.
2. Research Institute for Healthcare Policy Korean Medical Association. Analysis of the OECD Health Data 2006. 2007. 1st ed.
3. Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Eng J Med. 2001. 344:1343–1350.

4. Knowler WC, Barrett-Conner E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Eng J Med. 2002. 346:393–403.

5. Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997. 20:537–544.

6. Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract. 2005. 67:152–162.

7. Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003. 290:486–494.

8. DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators. Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006. 368:1096–1105.

9. Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004. 27:155–161.
10. American Diabetes Association. Standards of Medical Care in Diabetes-2008. Diabetes Care. 2008. 31:S12–S54.
11. Stratton IM, Adler AI, Andrew H, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000. 321:405–412.

12. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005. 353:2643–2653.

13. Taskforce Team for treatment guideline for diabetes. Treatment Guideline for Diabetes. 2007. 1st ed. Seoul: Korean Diabetes Association;40–44.
14. Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial. ACCORD Blood Sugar Treatment Strategy Announcement. 2008. accessed 2008 Jun 6. http://www.nhlbi.nih.gov/health/prof/heart/other/accord/.
15. Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG. Friedewald WTEffects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008. 358:2545–2559. Epub 2008 Jun 6.
16. ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008. 358:2560–2572. Epub 2008 Jun 6.
17. Welschen LM, Bloemendal E, Nijpels G, Dekker JM, Heine RJ, Stalman WA, Bouter LM. Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin: a systematic review. Diabetes Care. 2005. 28:1510–1517.

18. Towfigh A, Romanova M, Weinreb JE, Munjas B, Suttorp MJ, Zhou A, Shekelle PG. Self-monitoring of blood glucose levels in patients with type 2 diabetes mellitus not taking insulin: a meta-analysis. Am J Manag Care. 2008. 14:468–475.
19. Garg S, Zisser H, Schwartz S, Bailey T, Kaplan R, Ellis S, Jovanovic L. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care. 2006. 29:44–50.

20. Chetty VT, Almulla A, Odueyungbo A, Thabane L. The effect of continuous subcutaneous glucose monitoring (CGMS) versus intermittent whole blood finger - stick glucose monitoring (SBGM) on hemoglobin A1c (HBA1c) levels in Type I diabetic patients: a systematic review. Diabetes Res Clin Pract. 2008. 81:79–87. Epub 2008 Apr 15.

21. Taskforce Team for treatment guideline for diabetes. Treatment Guideline for Diabetes. 2007. 1st ed. Seoul: Korean Diabetes Association;44–45.
22. UK Prospective Diabetes Study Group. Response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients (UKPDS 7). Metabolism. 1990. 39:905–912.
23. Taskforce Team for treatment guideline for diabetes. Treatment Guideline for Diabetes. 2007. 1st ed. Seoul: Korean Diabetes Association;56–67.
24. Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. Management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: Update regarding thiazolidinediones: A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2008. 31:173–175.

25. Bell DS. Triple oral therapy for type 2 diabetes. Diabetes Res Clin Pract. 2007. 78:313–315. Epub 2007 Feb 22.

26. Roberts VL, Stewart J, Issa M, Lake B, Melis R. Triple therapy with glimepiride in patients with type 2 diabetes mellitus inadequately controlled by metformin and a thiazolidinedione: results of a 30-week, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2005. 27:1535–1547.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download