Abstract
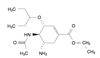
Oseltamivir (Tamiflu, the trade name) is a neuraminidase inhibitor used for the treatment and prevention of influenza virus infection as well as those of avian influenza infection. The mechanism of action is inhibition of neuraminidase for blocking the release of newly formed virus particles from the infected respiratory epithelial cells and preventing the further spread of the virus. Development of resistance is rare. The adult dose is 75mg (p.o.) b.i.d. for 5 days for treatment of influenza. The adverse drug reactions of oseltamivir are very rare but in children and adolescents careful monitoring is needed.
Figures and Tables
References
1. Korean Medical Association. Korean Academy of Medical Sciences Material for Media. Avian Influenza (AI) Recommendations for People May 8, 2008.
2. The Korean Society of Infectious Diseases. Infectious Diseases. 2007. Seoul: Koonja Publishing.
4. Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Jong MD, Lochindarat S, Nguyen TK, Nguyen TH, Tran TH, Nicoll A, Touch S, Yuen KY. Writing Committee of the World Health Organization (WHO). Consultation on Human Influenza A/H5. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005. 353:1374–1385.
5. Writing committee of the second world health organization consultation on clinical aspects of human infection with avian influenza A(H5N1) virus. Update of avian influenza A (H5N1) virus infection in humans. N Engl J Med. 2008. 358:261–273.
6. Woo JH. Pneumonia and Clinical Understanding Ulsan UUP. 1998.
7. World Health Organization. Influenza pandemic preparedness plan. The role of WHO and guidelines for national and regional planning. 1999. 04. Geneva: Switzerland.
9. De Clercq E. Antiviral agents active against influenza A viruses. Nature reviews. 2006. 5:1015–1025.

11. Monto AS, McKimm-Breschkin JL, Macken C, Hampson AW, Hay A, Klimov A, Tashiro M, Webster RG, Aymard M, Hayden FG, Zambon M. Detection of influenza viruses resistant to neuraminidase inhibitors in global surveillance during the first 3 years of their use. Antimicrob Agents Chemother. 2006. 50:2395–2402.

12. Sheu TG, Deyde VM, Okomo-Adhiambo M, Garten R, Xu X, Bright R, Butler E, Wallis TR, Klimov AI, Gubareva LV. Surveillance for neuraminidase inhibitor resistance among human influenza A and B viruses circulating worldwide in 2004-2008. Antimicrob Agents Chemother. 2008. 06. 14. (Epub ahead of print).
13. Hayden FG, Atmar RL, Schilling M, Johnson C, Poretz D, Paar D, Huson L, Ward P, Mills RG. Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N Engl J Med. 1999. 341:1336–1343.

15. Centers for Disease Control and Prevention. Prevention and control of influenza. MMWR Recomm Rep. 2007. 56:(RR-6).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download