Abstract
Complex regional pain syndromes (CRPS, formerly reflex sympathetic dystrophy and causalgia) are neuropathic pain disorders of one or more extremities developing inadequately after traumas or lesions in the peripheral or central nervous system (CNS). However, CPRS may also develop spontaneously. CRPS is clinically characterized by sensory (pain, hyperalgesia, and allodynia), autonomic (disturbances of skin temperature, color change, and presence of sweating abnormalities), and motor (paresis, tremor, and dystonia) disturbances. There have been growing evidences for that CRPS is a systemic disease involving the CNS and peripheral nervous system. The diagnosis is mainly based on clinical symptoms and signs, so that it could be under- or over-diagnosed. However, careful clinical evaluation and additional tests should lead to an adequate diagnosis. The goal of treatment is to improve functions, relieve pain, and achieve remission. The early diagnosis and multidisciplinary treatments, which include pain management, rehabilitation, and psychological therapy, are the most important elements for solving the patients' problems.
Figures and Tables
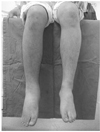 | Figure 1A CRPS patient with 7 months of pain duration. The patient suffered from the right leg pain, especially, below the ankle after the right knee contusion and arthroscopy. |
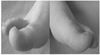 | Figure 2A CRPS patient with 8 months pain duration. Left foot shows reddish and swelling. Sweat drops are seen around the left great toe and right foot shows no sweating. |
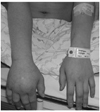 | Figure 3A CRPS patient with 4 months of pain duration. The patient developed CRPS of the right upper extremity after the right wrist sprain followed by falling down. Right hand shows severe swelling. |
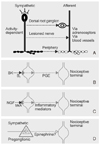 | Figure 4Possible couplings between sympathetic neurons and afferent neurons. Coupling with primary afferent neurons depends on activity in the sympathetic neurons and the expression of functional adrenoceptors by the afferent neurons, or is mediated indirectly via the blood vessels (blood flow). It can occur in the periphery, in the dorsal root ganglion, or, possibly, in the lesioned nerve (A). The inflammatory mediator bradykinin (BK) reacts with B2 receptors in the membrane of the sympathetic varicosities, inducing release of prostaglandin E2 (PGE2; B). Nerve growth factor (NGF) released during inflammation reacts with the high-affinity receptor trkA for NGF in the membrane of the sympathetic varicosities, inducing release of an inflammatory mediator or inflammatory mediators (C). Activation of the adrenal medulla (AM) by sympathetic preganglionic neurons leads to release of a hormone (possibly adrenaline) (D). (adopted from Janig w 2000). |
 | Figure 5Modes of neural plasticity at synapses onto nociceptive transmission neurons in the dorsal horn of the spinal cord. The neurons are activated by fast EPSPs and enhanced by slow EPSPs, plateau potentials, and windup. Modulation through intracellular kinase/phosphatase signaling cascades produces central sensitization through facilitating AMPA/kainate and NMDA receptor function or cell-surface expression. Modification is mediated by induced expression of gene products, loss of inhibitory interneurons, and establishment of aberrant excitatory synaptic connections (adopted from Woolf CJ 2003). |
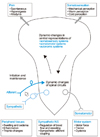 | Figure 6Schematic diagram of sensory, autonomic, and somatomotor changes in patients with CRPS I. Changes are triggered and possibly maintained by the nociceptive afferent input from the somatic and visceral body domains. Whether these central changes are reversible in patients with chronic CRPS I is unclear. The central changes possibly affect the endogenous control system of nociceptive impulse transmission. Coupling between the sympathetic neurons and the afferent neurons in the periphery (open arrow) is one component of the pain in patients with SMP. However, it seems to be unimportant in those without SMP. Note that SMP and pain that is not dependent on the sympathetic nervous system can exist in parallel in the same patient (adopted from Janig w 2000). |
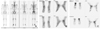 | Figure 7Three phase bone scan image of CRPS patient with pain duration of 13 months. Precipitating event was operation of right calcaneus fracture. Right ankle and whole metatarsophalangeal joints show hot uptake in blood pool and delayed image. |
 | Figure 9A CRPS patient suffering with 4 months of pain duration shows severe swelling of right hand (A). He had various interventional treatment including the sympathetic ganglion block as well as strong analgesics for 3 months, however his pain and swelling did not disappear. Spinal cord stimulation (SCS) was tried 5 months after the pain onset, his symptom was gradually improved. One month after the SCS his right hand swelling marked diminished (B) and he could get right hand grip (C). |
References
1. Mitchell SW, Morehouse GR, Keen WW. Gunshot wounds and other injuries of nerves. 1864. Clin Orthop Relat Res. 2007. 458:35–39.
2. Evan JA. Reflex sympathetic dystrophy. Surg Gynecol Obstet. 1946. 82:36–43.
3. Stanton-Hicks M, Janig W, Hassenbusch S, Haddox JD, Boas R, Wilson P. Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain. 1995. 63:127–133.

4. Allen G, Galer BS, Schwartz L. Epidemiology of complex regional pain syndrome: a retrospective chart review of 134 patients. Pain. 1999. 80:539–544.

5. Galer BS, Bruehl S, Harden RN. IASP diagnostic criteria for complex regional pain syndrome: a preliminary empirical validation study. Clin J Pain. 1998. 14:48–54.

6. Bruehl S, Harden RN, Galer BS, Saltz S, Bertram M, Backonja M, Gayles R, Rudin N, Bhugra MK, Stanton-Hicks M. External validation of IASP diagnostic criteria for complex regional pain syndrome and proposed diagnostic criteria. Pain. 1999. 81:147–154.

7. Harden RN, Bruehl S, Stanton-Hicks M, Wilson PR. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med. 2007. 8:326–331.

8. Harden RN, Bruehl S, Galer BS, Saltz S, Bertram M, Backonja M, Gayles R, Rudin N, Bhugra MK. Complex regional pain syndrome: are the IASP diagnostic criteria valid and sufficiently comprehensive? Pain. 1999. 83:211–219.

9. Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic painL the Neuropathic Pain Scale. Neurology. 1997. 48:332–338.

10. Price DD, Long S, Huitt C. Sensory testing of pathophysiological mechanisms of pain in patients with reflex sympathetic dystrophy. Pain. 1992. 49:163–173.

11. Sieweke N, Birklein F, Riedl B, Neundörfer B, Handwerker HO. Patterns of hyperalgesia in complex regional pain syndrome. Pain. 1999. 80:171–177.

12. Wasner G, Schattschneider J, Binder A, Baron R. Complex regional pain syndrome-diagnostic, mechanisms, CNS involvement and therapy. Spinal Cord. 2003. 41:61–75.

13. Park EJ, Han KR, Chae YJ, Jeong WH, Kim C. Cold stress thermography in the diagnosis of complex regional pain syndrome type 1. Korean J Pain. 2006. 19:159–163.

14. Deuschl G, Blumberg H, Lucking CH. Tremor in reflex sympathetic dystrophy. Arch Neurol. 1991. 48:1247–1252.

15. Park TJ, Martin GM, Magness JL, Kavanaugh GJ. Reflex sympathetic dystrophy. Review of 140 cases. Minn Med. 1970. 53:507–512.
16. Sudeck P. Über die acute entzündliche Knochenatrophie. Arch Kin Chir. 1900. 62:147–156.
17. Calder JS, Holten I, McAllister RM. Evidence for immune system involvement in reflex sympathetic dystrophy. J Hand Surg [Br]. 1998. 23:147–150.

18. van der Laan L, Goris RJ. Reflex sympathetic dystrophy. An exaggerated regional inflammatory response? Hand Clin. 1997. 13:373–385.
19. Renier JC, Bregeon C, Basle M, Seret P, Acquaviva P, Schiano A, Serratrice G, Amor B, May V, Delcambre B, D'Eshoughes JR, Vincent G, Ducastelle , Pawlotsky Y. The joint in algodystrophy. Joint fluid, synovium, cartilage. Rev Rhum Mal Osteoartic. 1983. 50:255–260.
20. Graif M. Synovial effusion in reflex sympathetic dystrophy: an additional sign for diagnosis and staging. Skeletal Radiol. 1998. 27:262–265.

21. Birklein F, Schmelz M, Schifter S, Weber M. The important role of neuropeptides in complex regional pain syndrome. Neurology. 2001. 91:251–257.

22. Huygen FJ, De Bruijin AG, Groeneweg JG, Klein J, Zijlstra FJ. Evidence for local inflammation in complex regional pain syndrome type 1. Mediators Inflamm. 2002. 11:47–51.

23. Baron R. Effect of sympathetic activity on capsaicin-evoked pain, hyperalgesia, and vasodilatiation. Neurology. 1999. 52:923–932.

24. Elam M, Olausson B, Skarphedinsson JO, Wallin BG. Does sympathetic nerve discharge affect the firing of polymodal C-fibre afferents in humans? Brain. 1999. 122:2237–2244.

25. Jänig W, Levine JD, Michaelis M. Interactions of sympathetic and primary afferent neurons following nerve injury and tissue trauma. Prog Brain Res. 1996. 113:161–184.
26. EcLachlan Em, Janig W, Devor M, Michaelis M. Perpheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature. 1993. 363:543–546.

27. Baron R, Levein JD, Fields HL. Causalgia and reflex sympathetic dystrophy: does the sympathetic nervous system contribute to the pain? Muscle Nerve. 1999. 22:678–695.

28. Baron R, Schattschneider J, Binder A, Siebrecht D, Wasner G. Relation between sympathetic vasoconstrictor activity and pain and hyperalgesia in complex regional pain syndromes: a case-control study. Lancet. 2002. 359:1655–1660.

29. Drummond PD, Finch PM, Skipworth S, Blockey P. Pain increases during sympathetic arousal in patients with complex regional pain syndrome. Neurology. 2001. 57:1296–1303.

30. Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999. 353:1959–1964.

31. Schwartzman RJ, Alexander GM, Grothusen J. Pathophysiology of complex regional pain syndrome. Expert Rev Neurother. 2006. 6:669–681.

32. Jänig W, Baron R. Complex regional pain syndrome: mystery explained? The Lancet Neurology. 2003. 2:687–697.

33. Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000. 288:1765–1769.

35. England S, Bevan S, Docherty RJ. PGE2 modulates the tetrodotoxin resistant sodium current in neonatal rat dorsal root ganglion neurons via the cyclic AMP-protein kinase A cascade. J Physiol. 1996. 495:429–440.

36. Ji RR, Kohno T, Moore KA. Central sensitization and LTP: do pain and memory share similar mechanism. Trends Neurosci. 2003. 26:696–705.

37. Kurvers Ha. Skin blood flow abnormalities in a rat model of neuropathic pain: result of decreased sympathetic vasoconstrictor outflow? J Auton Nerv Syst. 1997. 63:19–29.

38. Wakisaka S, Kajander KC, Bennett GJ. Abnormal skin temperature and abnormal sympathetic vasomotor innervations in an experimental painful peripheral neuropathy. Pain. 1991. 46:299–313.

39. Blumberg H, Jänig W. Reflex patterns in postganglionic vasoconstrictor neurons following chronic nerve lesions. J Auton Nerv Syst. 1985. 14:157–180.

40. Verdugo RJ, Ochoa JL. Abnormal movements in complex regional pain syndrome: assessment of their nature. Muscle Nerve. 2000. 23:198–205.

41. Ciccone DS, Bandilla EB, Wu W. Psychological dysfunction in patients with reflex sympathetic dystrophy. Pain. 1997. 71:323–333.

42. Van der Laan L, van Spaendonck K, horstink MW, Goris RF. The symptom checklist-90 revised questionnaire: no psychological profiles in complex regional pain syndrome-dystonia. J Pain Symptom Manage. 1999. 17:357–362.
43. Geertzen JH, de Bruijn-Kofman AT, de Bruijn HP, van de Wiel HB, Dijkstra PU. Stressful life events and psychological dysfunction in complex regional pain syndrome type I. Clin J Pain. 1998. 14:143–147.

44. Monti DA, Herring CL, Schwartzaman RJ. Personality assessment of patients with complex regional pain syndrome type I. Clin J Pain. 1998. 14:295–302.

45. Bruehl S, Husfeldt B, Lubenow TR, Nath H, Ivankovich AD. Psychological differences between reflex sympathetic dystrophy and non-RSK chronic pain patients. Pain. 1996. 67:107–114.

46. Bruehl S, Husfeldt B, Lubenow TR, Nath H, Ivankovich AD. Psychological differences between reflex sympathetic dystrophy and non-RSK chronic pain patients. Pain. 1996. 67:107–114.

47. Stanton-Hicks MD, Burton AW, Bruehl SP, Carr DB, Harden RN, Hassenbusch SJ, Lubenow TR, Oakley JC, Racz GB, Raj PP, Rauck RL, Rezai AR. An updated interdisciplinary clinical aptheway for CRPS: report of an expert panel. Pain Practice. 2002. 2:1–16.

48. Rowbotham MC, Reisner Keller LA, Fields HL. Both intravenous lidocaine and morphine reduce the pain of psotherpetic neuralgia. Neurology. 1991. 41:1024–1028.

49. Portenoy RK, Foley KM, Inturrisi CE. The nature of opioid responsiveness and its implications for neuropathic pain: a new hypothesis derived from studies of opioid infusions. Pain. 1990. 43:273–286.

50. Max MB, Schafer SC, Culnane M, Smoller B, Dubner R, Gracely RH. Amitriptyline, but not lorazepam, relieves postherpetic neuralgia. Neurology. 1988. 38:1427–1432.

51. Rintala DH, Holmes SA, Courtade D, Fiess RN, Tastard LV, Loubser PG. Comparison of the effectiveness of amitriptyline and gabapentin on chronic neuropathic pain in persons with spinal cord injury. Arch Phys Med Rehabil. 2008. 88:1547–1560.

52. Gore M, Sadosky A, Tai KS, Stacey B. A retrospective evaluation of the use of gabapentin and pregabalin in patients with postherpetic neuralgia in usual-care settings. Clin Ther. 2007. 29:1655–1670.

54. Mellick GA, Mellick LB. Gabapentin in the management of reflex sympathetic dystrophy. J Pain Sympt Manage. 1995. 10:265–266.

55. Zuniga RE, Perera S, Abram SE. Intrathecal baclofen: a useful agent in the treatment of well-established complex regional pain syndrome. Reg Anesth Pain Med. 2002. 27:90–93.

56. Bianchi C, Rossi S, Turi S, Brambilla A, Felisari G, Mascheri D. Long-term functional outcome measures in corticosteroid-treated complex regional pain syndrome. Eura Medicophys. 2006. 42:103–111.
57. Kiefer RT, Rohr P, Ploppa A, Nohe B, Dieterich HJ, Grothusen J, Altemeyer KH, Unertl K, Schwartzman RJ. A pilot open-label study of the efficacy of subanesthetic isomeric S(+)-ketamine in refractory CRPS patients. Pain Med. 2008. 9:44–54.

58. Correll GE, Maleki J, Gracely EJ, Muir JJ, Harbut RE. Subanesthetic ketamine infusion therapy: a retrospective analysis of a novel therapeutic approach to complex regional pain syndrome. Pain Med. 2004. 5:263–275.

59. Robbins WR, Staats PS, Levine J, Fields HL, Allen RW, Campbell JN, Pappagallo M. Treatment of intractable pain with topical large-dose capsaicin: preliminary report. Anesth Analg. 1998. 86:579–583.
60. Perez RS, Zuurmond WW, Bezemer PD, Kuik DJ, van Loenen AC, de Lange JJ, Zuidhof AJ. The treatment of complex regional pain syndrome type I with free radical scavengers: a randomized controlled study. Pain. 2003. 102:297–307.

61. Reuben SS, Sklar J. Intravenous regional anesthesia with clonidine in the management of complex regional pain syndrome of the knee. J Clin Anesth. 2002. 14:87–91.

62. Wallace MS, Ridgeway BM, Leung AY, Gerayli A, Yaksh TL. Concentration-effect relationship of intravenous lidocaine on the allodynia of complex regional pain syndrome types I and II. Anesthesiology. 2000. 92:75–83.

63. Cepeda MS, Lau J, Carr DB. Defining the therapeutic role of local anesthetic sympathetic blockade in complex regional pain syndrome: a narrative and systematic review. Clin J Pain. 2002. 18:216–233.





 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download