Abstract
Multiple Sclerosis (MS) is the most common demyelinating disease affecting the central nervous system of young adults living in western countries. The clinical suspicion of demyelination, as a pathological process, is high when a young adult develops one or more neurological episodes indicating a damage to white matter tracts within the central nervous system, especially when the optic nerve, brainstem, or spinal cord is involved. The patient with episodes disseminating in time, each of which can be attributed to demyelination, requires no investigation prior to establishing the diagnosis of clinically definite MS, if the age of clinical presentation falls between 20 and 50 years, if different anatomical sites within the central nervous system have necessarily been affected on separate occasions, and if the clinical manifestation is typical for MS. MS in Asian populations is characterized by selective and dominant involvement of the optic nerve and spinal cords, as well as some incidence of brainstem lesion and symptoms. About 35~40% of cases of MS in Korea are of this optico-spinal type. Optico-spinal MS (OSMS) generally has a higher female-to-male ratio than conventional MS. OSMS is also characterized by frequent relapses, severe disability, few brain lesions on MRI, and a very lower incidence of oligoclonal bands in CSF. Neuromyelitis Optica (NMO) (Devic syndrome), which causes severe optic neuritis (ON) and longitudinally extensive transverse myelits either with a monophase or relapse-remitting pattern, is rare in Korean. NMO-IgG was reported to be helpful for the diagnosis of early-stage NMO and differential diagnoses of MS.
Figures and Tables
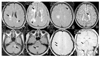 | Figure 1Multiple sclerosis lesions (arrows) depicted using (A~F) fast FLAIR, and (G, H)T1-weighted gadolinuim enhanced sequence |
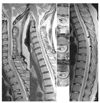 | Figure 2Multiple sclerosis. (A)~(C)T2-weighted Saggital MRI and (B-1,2,3) axial MRI of the spinal cord shows multiple small to medium intrinsic lesions, which involve only part of the cord cross-section and extend to the surface of the cord(arrows) |
Table 2
Diagnostic Criteria for neuromyelitis optica (3)
Diagnosis requires all absolute criteria and one major supportive criterion or two minor supportive criteria

References
2. Devic E. Myelite subaigue compliquee de neurite optique. Bull Med. 1894. 8:1033–1034.
3. Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology. 1999. 53:1107–1114.

4. Swingler RJ, Compston DAS. The clinical features of multiple sclerosis in south east Wales. Q J Med. 1992. 83:325–337.
5. Kim KK. Optico-spinal multiple sclerosis and neuromyelitis optica. J Kor Neurol Assoc. 2006. 24:S. 66–69.
6. Kira J, Kanai T, Nishimura Y, Yamasaki K, Matsushita S, Kawano Y, Hasuo K, Tobimatsu S, Kobayashi T. Western versus Asian types of multiple sclerosis: immunogenetically and clinically distinct disorders. Ann Neurol. 1996. 40:569–574.

7. Shibasaki H, McDonald WI, Kuroiwa YJ. Racial modification of clinical picture of multiple sclerosis; comparison between British and Japanese patients. J Neurol Sci. 1981. 49:253–271.
8. Yu YL, Woo E, Hawkins BR, Ho HC, Huang CY. Multiple sclerosis amongst Chinese in Hong Kong. Brain. 1989. 112:1445–1467.

9. Yu YL, Woo E, Hawkins BR, et al. Multiple sclerosis among Chinese in Hong Kong. Brain. 1989. 112:1445–1467.
10. Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. JEM. 2005. 202:473–477.

11. Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004. 364:2106–2112.

12. Nakashima I, Fujihara K, Miyazawa I, Misu T, Narikawa K, Nakamura M, et al. Clinical and MRI features of Japanese MS patients with NMO-IgG. JNNP. 2006.
13. Compston A, editor. McAlpine's Multiple Sclerosis: The diagnosis of multiple sclerosis. 2006. 4th ed. Churchill Livingston;351–388.
14. Miller DH, Rudge P, Johnson G, Kendall BE, Macmanus DG, Moseley IF, Barnes D, McDonald WI. Serial gadolinium enhanced magnetic resonance imaging in multiple sclerosis. Brain. 1988. 111:927–939.

15. Cotton F, Weiner HL, Jolesz FA, Guttmann CR. MRI contrast uptake in new lesions in relapsing-remitting MS followed at weekly intervals. Neurology. 2003. 60:640–646.

16. Kidd D, Thorpe JW, Kendall BE, Barker GJ, Miller DH, Mc-Donald WI, Thompson AJ. MRI dynamics of brain and spinal cord in progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 1996. 60:15–19.

17. Thompson AJ, Kermo AG, Wicks D, MacManus DG, Kendall BE, Kingsley DP, McDonald WI. Major differences in the dynamics of primary and secondary progressive multiple sclerosis. Ann Neurol. 1991. 29:53–62.

18. van Waesberghe JH, Kamphorst W, De Groot CJ, van Walderveen MA, Castelijns JA, Ravid R, Lycklama a, van der Valk P, Polman CH, Thompson AJ, Barkhof F. Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol. 1999. 46:747–754.

19. Uhlenbrock D, Sehlen S. The vaule of T1-weighted images in the differentiation between MS, white matter lesions and subcortical arteriosclerotic encephalopathy (SAE). Neuroradiology. 1989. 31:203–212.

20. Bagnato F, Jeffries N, Richert ND, Stone RD, Ohayon JM, Mc-Farland HF, Frank JA. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003. 126:1782–1789.

21. McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sandberg-Wollheim M, Sibley W, Thompson A, van den Noort S, Weinshenker BY, Wolinsky JS. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001. 50:121–127.

22. Compston A, editor. McAlpine's Multiple Sclerosis: Disease-modifying treatments in multiple sclerosis. 2006. 4th ed. Churchill Livingston;729–810.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download