Abstract
An Escherichia coli strain (SEPT13) isolated from the liver of a hen presenting clinical signs of septicaemia had a LD50 of 4.0 × 105 CFU/ml in one-day-old chickens, expressed Ia, Ib, E1, E3, K and B colicins and aerobactin. The strain was ampicillin and streptomycin resistant, and found to have fimA, csgA and tsh DNA related sequences; it could adhere to and invade HEp-2 and tracheal epithelial cells, expressed fimbriae (observed by electron microscopy), and had five plasmids of 2.7, 4.7, 43, 56, and 88 MDa. Transposon mutagenesis of strain SEPT13, with transposon TnphoA, resulted in a mutant strain named ST16 that had a LD50 of 1.2 × 1012 CFU/ml. All other biological characteristics of strain ST16 were the same as those detected for strain SEPT13 except for the migration of an 88 MDa plasmid to the 93 MDa position indicating the insertion of the transposon into the 88 MDa plasmid. The 93 MDa plasmid of strain ST16 was transferred, by electroporation assay, to non-pathogenic receptor strains (E. coli strains K12 MS101 and HB101), resulting in transformant strains A and B, respectively. These strains exhibited adhesion properties to in vitro cultivated HEp-2 cells but did not have the capacity for invasion. The adherence occurred despite the absence of fimbriae; this finding suggests that the 88 MDa plasmid has afimbrial adhesin genes.
Escherichia coli is frequently found as a normal inhabitant of the intestinal tract of humans and animals. However, some strains, capable of causing disease, are pathogenic clones in healthy hosts [23]. Avian pathogenic E. coli strains (APEC) are most commonly associated with extraintestinal infections, mainly in the respiratory tract or systemic infections; a variety of diseases can result, which are responsible for severe economic losses in the avian industry [11,17,18].
The pathogenesis and the role of virulence present in APEC strains have not been fully elucidated to date. However, considerable progress has been made recently to establish the mechanisms of pathogenesis [11]. Flagella, toxins and cytotoxins, serum resistance, colicin production, iron sequestering systems, temperature-sensitive hemagglutinin and expression of adhesins, are considered to be the fundamental virulence associated factors for the full expression of APEC pathogenecity [5,9,10,12,36]. Expression of adhesins was first detected by the observation that a virulent and fimbriated strain was less easily cleared from the trachea of turkeys than a non-virulent and less-fimbriated strain [1]. The principal adhesins described for APEC strains are type 1, type P, curli fimbriae and temperature-sensitive hemagglutinin (Tsh). Type 1 and type P fimbriae are encoded by the fim and pap gene clusters, respectively, that are located on the E. coli chromosome [28]. Curli fimbriae have been associated with bacterial adherence to laminin and fibronectin [26] and with chicken red blood cell agglutination, but their involvement in pathogenesis is still unclear and remains to be clarified [27]. The tsh gene, which encodes a Tsh, was first identified by Provence and Curtis III [30] and was shown to be associated with APEC but not with E. coli isolated from the feces of healthy chickens; [22] this suggested that hemagglutinin could be associated with APEC pathogenesis.
There is strong evidenc that adhesion properties are associated with APEC pathogenicity. The purpose of this stud was to determine the association of pathogenicity and adhesion characteristics expressed by an avian septicaemic E. coli strain (SEPT13) and to correlate these characteristics with the presence of the 88 MDa plasmid found in this strain. In addition, we compared these results with previous reports on strain SEPT13. Furthermore, once the genetic location of the adhesin operon is determined it could be cloned and expression of the adhesion protein could be studied to improve our understanding of the role of adhesion in Brazilian chicken flocks.
Escherichia coli strain SEPT 13 was isolated from the liver of a chicken with clinical signs of septicaemia. The E. coli strains K12 MS101 (nalidixic acid resistant) and HB101 (streptomycin resistant) are non-pathogenic strains that were used as recipient strains for transformation experiments using the electroporation technique. E. coli strain LG 1522 [6] was used as an indicator strain for aerobactin production. E. coli strains R80 (all colicins), R81 (col I), R82 (col Ia), R83 (col Ib), R675 (col E1), R676 (col E3), R914 (col ROW-K), R915 (col V), and R996 (col B) were used as indicator strains for specific colicins. They were a gift from Dr. E. C. Souza, at the Federal University of Minas Gerais at Belo Horizonte, MG. E. coli V517 is a strain that harbors plasmids of different sizes (32, 5.12, 3.48, 3.03, 2.24, 1.69, 1.51, and 1.25 MDa); [20] they were used as molecular standards in the agarose gel electrophoresis. Plasmid pRT733 [43] containing transposon TnphoA was used for the mutagenesis experiments. LB and LA media [34] were used for routine bacterial growth. All strains were stored in LB medium containing 15% glycerol at -70℃ to avoid the loss of plasmids.
The resistance of antimicrobial drugs (ampicillin, kanamycin, streptomycin, tetracycline, and chloramphenicol) was determined as described by Chulasari and Suthienkul [8]. Concentrations of 5, 10, 25, 50, 100, 250, and 500 µg/ml were used to determine the resistance level for each antibiotic. The maximum concentration of an antibiotic that still had bacterial growth was considered the minimal inhibitory concentration for that antibiotic.
Pathogenicity assays were performed as described by Fantinatti et al. [14]. Briefly, a 1.0 ml suspension (LB medium, 37℃, 14-18 h; washed twice with and resuspended in 0.85% sterilized saline solution) of the strain to be tested was diluted ten-fold (10-1 to 10-11) and 0.5 ml of each dilution was injected subcutaneously into the neck region of groups of six one-day-old-male chickens. These groups were observed throughout a 7-day period. The LD50 was calculated by the method of Reed and Muench [32] for each strain. All of the experiments were conducted with germ-free white leghorn chickens. Each group of animals was separated into cages that were cleaned daily and fed ad libido with sterile water and food.
Colicin was produced as described by Azevedo and da Costa [3]. Briefly, the strains were cultured overnight in LB medium at 37℃ and a drop was plated onto LA agar. After the overnight incubation at 37℃ all bacterial growth was destroyed by chloroform fumes and then overlaid with 3.0 ml of soft LA medium containing a colicin-indicator strain. The capacity for colicin production (Ia, Ib, E1, E3, K, and B) was determined by the presence of a clear halo around the destroyed bacterial colonies after an overnight incubation period.
Aerobactin production was assayed by the method of Carbonetti and Williams [6] using E. coli LG 1522 as the indicator strain. For this purpose, symmetric holes were made in the LA medium containing 200 µM α-α-dipyridyl and then filled with the supernatant of the bacterial growth (iron-free LB medium, 37℃, overnight) of each strain to be tested. Once the medium had absorbed all of the liquid, strain LG 1522 was inoculated onto its surface and the Petri dish incubated at 37℃. Growth of LG 1522 colonies, over 72 h, around a given hole, indicated the capacity of that strain to produce aerobactin.
The capacity for adhesion and invasion of all strains into HEp-2 cells was studied as described by Scaletsky et al. [35] and Vidotto et al. [44], with slight modifications. Briefly, cultures of these cells were grown in 24-well tissue culture microplates (BD Falcon, USA) where sterile round cover slips (13 mm in diameter) were placed prior to the inoculation with the cells. The growth medium for each microplate well consisted of 0.9 ml of Eagle's minimal essential medium (MEM) with 10% fetal calf serum, 1% D-mannose, and 1% antibiotics solution (penicillin 100,000 U and streptomycin 100 µ/ml). The microplates were incubated in 10% CO2 atmosphere at 37℃ until a semi-confluent monolayer was formed. Afterwards, the monolayers were washed 3 times with sterile phosphate buffered saline (PBS) 0.05 M, pH 7.2. Then, 0.1 ml aliquots of the bacterial culture (37℃ -18 h, in LB medium) containing 2 × 107 colony forming units (CFU) were added to the wells. After 3 h of incubation at 37℃, the monolayers were washed 10 times with PBS buffer, fixed with methanol for 10 min, stained with the May-Grunwald and Giemsa stains, and observed under bright field microscopy (×1,000).
Adhesion to tracheal epithelial cells was evaluated as described by Dho and Lafont [9] and Pourbakhsh et al. [29] using 18-day avian SPF (specific pathogen free) embryonated eggs. Briefly, the trachea was aseptically removed from 18 day avian embryos, rinsed in PBS (pH 7.4), and cut in 5 mm sections. Adherence studies were performed in the 96-well-round-bottom microtiter plates, as described below: two trachea rings and 25 µl of Eagle medium with 5% calf serum were placed into each well. A suspension of each bacterium (109 cells/ml) previously grown on LB (37℃ - 18 h) was incubated with the tracheal rings at 37℃ for 30 min, after which they were washed with PBS and incubated for 4 h (37℃). The tracheal sections were rinsed with PBS-formalin. The tracheal rings were dehydrated, xylol treated and blocked with paraffin. Five µm thick sections were cut using a microtome, mounted on glass slides, hydrated and stained with Giemsa. The adherence assay was performed in the presence and in the absence of 1% D-mannose.
Plasmid DNA was extracted as described by Sambrook et al. [34] and suspended in sterilized deonized water and stored frozen until use. The plasmid DNA to be used in the electroporation experiments was cleaned using the Wizard DNA Clean-up columns (Promega, USA). Plasmid DNA electrophoresis and ethidium bromide staining of the gels were carried out as described by Sambrook et al. [34].
The electroparation assays were performed as described by Dower et al. [13] with minor modifications. For this, the recipient strains were grown in LB medium (50 ml, 37℃, 150 rpm) until an absorbance of 0.5, at a wavelength above 500 nm. Then, they were extensively washed with iced 10% (10 ml) glycerol and resuspended in 100 µl of the washing solution. Next, 60 µl of the suspension was electroporated (2,500 V; 800 ohms of resistance; 25 µF of capacitance in 15.3 sec) with 20 µl of the plasmid DNA suspension in a Gene Pulser II (Bio-Rad, USA). Transformant strains were selected on the LA medium containing specific antibiotic markers for the recipient strains and the electroporated plasmid DNA.
Transposon mutagenesis (TnphoA) was accomplished as described by Taylor et al. [43] using plasmid pRT733. Mutants were obtained on LA medium containing 40 µg/ml of 5-bromo-4-chloro-3-indolyl phosphate and selective antibiotics. Blue, kanamycin resistant colonies were analyzed by agarose gel electrophoresis to establish the plasmid DNA profiles. All strains that presented with an increased plasmid size, as observed by agarose gel electrophoresis, were tested for the LD50 using a method described previously.
A 3,450 bp DNA fragment of transposon TnphoA was cut from the plasmid pRT733 using the restriction enzyme Bst EII, and then purified from the agarose gel using the dialysis method as described by Sambrook et al. [34]. This fragment was labeled using the Alk-Phos kit (Amersham Pharmacia, Sweden), and then hybridized with plasmidial DNA (88 MDa mutagenized plasmid) fragments that were obtained after treatment with the restriction enzymes Eco RI, Eco RV and Bst EII; the fragments were separated by agarose gel electrophoresis as described by Sambrook et al. [34].
The Electronic Microscopy was carried out as described by Sperandio and Silveira [39]. For this purpose, the bacterial strain was grown in LB medium at 37℃, overnight. After centrifugation (13,000 × g; 30 sec), the pellet was resuspended in 200 µl of milli-Q water and 10 µl of this growth was mixed and fixed with 1% phosphotungstic acid for 30 sec. This bacterial suspension was added onto a 400 mesh grid coated with Formvar; the grids were dried in a carbon-evaporator and observed using a transmission electronic microscope (LEO 906; LEO Elektronenmikroskopie, Germany).
A total of 20 ng of genomic bacterial DNA was extracted as described by Ausubel et al. [2] and resuspended in TE buffer plus 10 mg/ml RNAse and used for PCR. The primers used for the amplification of the pathogenicity related sequences and the PCR conditions were the same as those described by the authors cited in Table 1. All amplification reactions were performed in a Mastercycle termocycle (Eppendorf, Germany). The PCR products were analyzed by electrophoresis in a 1.0% submersed agarose gel stained with ethidium bromide and visualized under UV light as described by Sambrook et al. [34].
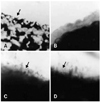
Table 2 shows the biological characteristics of the wild type SEPT 13 strain and its derivative strains. SEPT 13 is an APEC (wrinkled) strain that was isolated from a chicken with clinical signs of septicaemia. It expresses colicins Ia, Ib, E1, E3, K and B; it produces aerobactin and is resistant to ampicillin, tetracycline and streptomycin. In addition, it harbors five different plasmids 2.7, 4.7, 43, 56, and 88 MDa (Fig. 1, Lane 2). This strain demonstrated a D-mannose resistant diffuse adhesion to HEp-2 cells cultivated in vitro (Fig. 2A), an adherence to tracheal epithelial cells (Fig. 3A) and was able to invade HEp-2 cells (Table 2). Fimbriae expression was detected when this strain was studied under an electron microscope (Fig. 4A). In the one-day-old chicken assay the LD50 of strain, SEPT 13 was determined to be 4.0 × 105 CFU/ml (Table 2). The PCR experiments demonstrated, in this strain only, the presence of fimA, csgA and tsh genes, and was negative for all the other genes as noted in Table 1 (data not shown).
Mutagenesis of the SEPT 13 strain with transposon TnphoA (Kmr, alcaline phosphatase gene) resulted in 12 mutant strains. Agarose gel electrophoresis of these strains demonstrated that the transposon TnphoA had been inserted into the 88 MDa plasmid that was increased in size (93 MDa), in three of these transformant strains. These strains where evaluated by the LD50 pathogenicity assay; one of them was found to have a decrease in pathogenicity (LD50 of 1.2 × 1012 CFU/ml) (Table 2). This mutant strain was termed strain ST16. In addition to the decreased pathogenicity, all other biological characteristics were present in the mutant ST16 (Table 2). To characterize the biological characteristics of the 93 MDa plasmid, a total plasmidial DNA preparation of strain ST16 was electroporated into strains MS101 (non-pathogenic, nalidixic acid resistant) and HB101 (a non-fimbriated, non-pathogenic, streptomycin resistant). Although of different genetic backgrounds, both of the recipient strains are adhesion and invasion negative to HEp-2 cells. The transformant strains containing only the 93 MDa plasmid (corresponding to the 88MDa plasmid carrying the transposon TnphoA), as determined by agarose gel electrophoresis, were selected in the LB plates with Km, resulting in the transformant strains A (Fig. 1) and B (data not shown), derived from strains MS101 and HB101, respectively. Hybridization experiments using a 3,450 bp Bst EII fragment of transposon TnphoA as a molecular probe confirmed the insertion of TnphoA into the 93 MDa plasmid (data not shown).
Strains A and B were unable to produce colicin or aerobactin, were invasion negative for HEp-2 cells (data not shown) but had mannose resistant adhesion to this cell type (Figs. 2D and F, respectively). In this assay, the wild type strains MS101 (Fig. 2B) and HB101 (Fig. 2E) were non-adherent. On the other hand, and as previously pointed out, SEPT 13 and the isogenic mutant strain ST 16 presented with a diffuse adherence pattern (Figs. 2A and C, respectively). The PCR assay was unable to amplify any of the genes that were previously detected in the SEPT 13 strain (tsh, csgA, and fimA) using the genomes of transformants A and B as templates. In addition, strains A and B, as well as mutant ST16, had a LD50 of more than 1011 CFU/ml (Table 2) when evaluated by the one-day-old chicken assay.
The adherence of strains, onto the tracheal epithelial cells, was also assayed (Fig. 3). As expected, the MS101 strain was non-adherent (Fig. 3B). On the other hand, SEPT 13, ST16 and transformant A were adherent to the tracheal epithelial cells (Figs. 3A, B and D, respectively). Transformant B was not tested in this assay.
Electron microscopy studies were performed with strains SEPT13, ST16, transformant A, B, MS101, and HB101. With the exception of transformant B and HB101, all other strains, including the receptor strain MS101, exhibited fimbriae on their surface, as noted in Figure 4. Despite the absence of fimbrial structures on the surface of transformant B (Fig. 4C), this strain was able to adhere to HEp-2 cells (Fig. 2F), in contrast to the results exhibited by the HB101 recipient strain (Fig. 2E).
The aim of this study was to correlate the presence of a high-molecular weight plasmid (88 MDa) with virulence and the biological traits of strain SEPT 13. For this, strain SEPT 13 was transposon-mutagenised resulting in a less virulent strain (strain ST 16).
In a previous study performed by Stehling et al. [40], a 43 MDa plasmid present in SEPT 13 was transferred to a recipient strain that resulted in a transformant called transformant E that expressed fimbriae and harbored the gene tsh. This gene was proposed as a candidate responsible for the adhesion and invasion characteristics of strain SEPT 13. They demonstrated that this plasmid (43 MDa) was not associated with the major factors responsible for pathogenicity in strain SEP 13 as observed in the one-day-old chicken assay. This is because the transfer of the plasmid to the recipient strains did not increase virulence.
The results of the mutagenesis experiments herein accomplished suggest that the 88 MDa plasmid might be responsible, at least in part, for the pathogenicity observed in strain SEPT 13. This is because strain ST16 was less virulent than SEPT 13 and had the insertion of the transposon in this plasmid as indicated by the plasmid profile and hybridization experiments. Previous studies [25,38,45] have also indicated that high-molecular weight plasmids could have genes involved in the pathogenicity of avian E. coli strains.
Transformant A exhibited fimbriae expression and adhesion to HEp-2 and chicken embryo tracheal cells, but was unable to invade the HEp-2 cells. The fimbriae expressed by this transformant cell, might have been expressed by the MS101 strain; therefore, we transferred the plasmid to a non-fimbriated strain (HB101). As a result, no fimbriae were expressed by the new transformant strain (transformant B). However, the transformant strains (A and B) had adhesion characteristics identified in the HEp-2 cells. This indicates that these strains were expressing adhesin that was not expressed by strains MS101 and HB101. In addition, transformant A was able to adhere to chicken embryo tracheal epithelial cells, unlike strain MS101. These results suggest that afimbrial adhesins were encoded by genes present in the 88 MDa plasmid, and likely responsible for the adhesion characteristics of the transformant strains. In addition, the insertion of transposon TnphoA in the 88 MDa plasmid did not knock out the adhesion genes. Although strain SEP13 harbors fimA, tsh and csgA genes, as detected by PCR, they were not responsible for the observed adhesion in the transformed strains since they were not transferred to these strains.
Our results support those of Stehling et al. [40]; in that the SEPT13 strain was found to have, a 43 MDa plasmid, with genes responsible for the expression of fimbrial adhesins that are responsible for the adhesion and invasion properties observed in this strain. In addition, this strain appears to have afimbrial adhesin genes located in the 88 MDa plasmid responsible for the adhesion properties herein studied. These observations suggest that the SEPT 13 strain has more than one adhesin type responsible for all adhesion properties; as demonstrated by adhesion genes expressed in different plasmids. The attenuation of virulence exhibited by the mutant ST16 in the one-day-old chicken assay is remarkable. Considering that the invasion capacity is mediated by the 43 MDa plasmid, we speculate that the 88 MDa plasmid may carry genes related to serum resistance or in vivo replication, abilities that almost invariably are exhibited by bacteria that cause systemic infections. Therefore, insertion mutagenesis of the 88 MDa plasmid, mediated by the TnphoA transposon, probably impaired the function of the genes essential for mediation or regulation of the expression of such characteristics. However, further studies are needed to characterize these
genes.
In conclusion, the results of this study show that a 88 MDa plasmid has genes responsible for adhesion in avian pathogenic E. coli to in vitro cultured cells and to tracheal epithelial cells. These adhesion characteristics are likely mediated by a non-fimbriated adhesin. In addition, this plasmid probably carries genes or operons essential for the pathogenicity observed in the one-day-old chicken assay, which requires additional study. These results, together with those obtained in a previous work conducted by our research group [40] indicate that the pathogenesis of APEC is very complex and further investigations are necessary to improve our understanding of it. In addition, the recognition that these strains express more than one adhesin suggest that molecular cloning of these compounds may help improve our understanding of the pathogenicity of avian Escherichia coli.
Figures and Tables
Fig. 1
Agarose gel electrophoresis (0.7%) of plasmid DNA from the SEPT13 strain, its derivative transformant strains and the reference plasmids Lane 1: Strain V517 (32 MDa), Lane 2: Strain SEPT13, Lane 3: Strain ST16, Lane 4: Recipient strain MS101 harboring the 93 MDa plasmid (Strain transformant A).
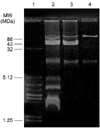
Fig. 2
Adhesion of strain SEPT13 and its derivative transformant strains to Hep-2 cells. (A) Strain SEPT 13; (B) Strain MS101 (C) Strain ST16; (D) Strain transformant A; (E) Strain HB101; (f) Strain transformant B. ×1,000.
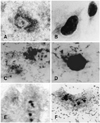
Fig. 3
Adhesion of strains SEPT 13 and its derivative transformant strains to tracheal epithelial cells. (A) Strain SEPT 13; (B) Recipient strain MS101; (C) Strain ST16; (D) Recipient strain MS101 harboring the 93 MDa plasmid (Strain transformant A). Arrowheads identify bacterial cells adherent to the tracheal epithelial cells. ×1,000.
Acknowledgments
This work was supported by Grants No. 96/03683-0 and 99/05830-2 from the Foundation for the Support of Research of the State of Sao Paulo (FAPESP) and by Grant No. 300121/90-3 from the National Council for Scientific and Technological Development (CNPq).
References
1. Arp LH, Jensen AE. Piliation, hemagglutination, motility, and generation time of Escherichia coli that are virulent or avirulent for turkeys. Avian Dis. 1980. 24:153–161.

2. Ausubel FM, Brente R, Kingston RE, Moore DD, Smith JA, Seidman JG, Struhl K. Cur Prot Mol Biol Green Publishing Associates. 1988. Brooklyn, NY:
3. Azevedo JL, da Costa SOP. Exercícios Práticos de Genética. 1973. São Paulo: EDVSP;171–174.
4. Blanco M, Blanco JE, Alonso MP, Mora A, Balsalobre C, Muñoa F, Juárez A, Blanco J. Detection of pap, sfa and afa adhesin-encoding operons in uropathogenic Escherichia coli strains: relationship with expression of adhesins and production of toxins. Res Microbiol. 1997. 148:745–755.

5. Campos TA, Stehling EG, Ferreira A, Castro AFP, Brocchi M, Silveira WD. Adhesion properties, fimbrial expression and PCR detection of adhesin-related genes of avian Escherichia coli strains. Vet Microbiol. 2005. 106:275–285.
6. Carbonetti NH, Williams PH. A cluster of five genes specifying the aerobactin iron uptake system of plasmid ColV-K30. Infect Immun. 1984. 46:7–12.

7. Clermont O, Bonacorsi S, Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol. 2000. 66:4555–4558.

8. Chulasiri M, Suthienkul O. Antimicrobial resistance of Escherichia coli isolated from chickens. Vet Microbiol. 1989. 21:189–194.
9. Dho M, Lafont JP. Escherichia coli colonization of the trachea in poultry: comparison of virulent and avirulent strains in gnotoxenic chickens. Avian Dis. 1982. 26:787–797.

10. Dho M, Lafont JP. Adhesive properties and iron uptake ability in Escherichia coli lethal and lethal for chicks. Avian Dis. 1984. 28:1016–1025.

11. Dho-Moulin M, Fairbrother JM. Avian pathogenic Escherichia coli (APEC). Vet Res. 1999. 30:299–316.
12. Dozois CM, Daigle F, Curtiss R III. Identification of pathogen-specific and conserved genes expressed in vivo by an avian pathogenic Escherichia coli strain. Proc Natl Acad Sci U S A. 2003. 100:247–252.

13. Dower WJ, Miller JF, Ragsdale CW. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988. 16:6127–6145.

14. Fantinatti F, Silveira WD, Castro AFP. Characteristics associated with pathogenicity of avian septicemic Escherichia coli strains. Vet Microbiol. 1984. 41:74–86.
15. Fields PI, Blom K, Hughes HJ, Helsel LO, Feng P, Swaminathan B. Molecular characterization of the gene encoding H antigen in Escherichia coli and development of a PCR-restriction fragment length polymorphism test for identification of E. coli O157:H7 and O157:NM. J Clin Microbiol. 1997. 35:1066–1070.

16. Gannon VP, Rashed M, King RK, Thomas EJ. Detection and characterization of the eae gene of Shiga-like toxin-producing Escherichia coli using polymerase chain reaction. J Ciln Microbiol. 1993. 31:1268–1274.

17. Gross WB. Gyles CL, editor. Diseases due to Escherichia coli in poultry. Escherichia coli in Domestic Animals and Humans. 1994. 1st ed. Wallingford: CAB International;237–259.
18. La Ragione RM, Woodward MJ. Virulence factors of Escherichia coli serotypes associated with avian colisepticaemia. Res Vet Sci. 2002. 73:27–35.

19. Le Bouguenec C, Archambaud M, Labigne A. Rapid and specific detection of the pap, afa, and sfa adhesin-encoding operons in uropathogenic Escherichia coli strains by polymerase chain reaction. J Clin Microbiol. 1992. 30:1189–1193.

20. Macrina FL, Kopecko DJ, Jones KR, Ayers DJ, McCowen SM. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978. 1:417–420.

21. Marc D, Dho-Moulin M. Analysis of the fim cluster of an avian O2 strain of Escherichia coli: serogroup-specific sites within fimA and nucleotide sequence of fimI. J Med Microbiol. 1996. 44:444–452.

22. Maurer JJ, Brown TP, Steffens WL, Thayer SG. The occurrence of ambient temperature-regulated adhesins, curli, and the temperature-sensitive hemagglutinin Tsh among avian Escherichia coli. Avian Dis. 1998. 42:106–118.

23. Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998. 11:142–201.
24. Nicholls L, Grant TH, Robins-Browne RM. Identification of a novel genetic locus that is required for in vitro adhesion of a clinical isolate of enterohaemorrhagic Escherichia coli to epithelial cells. Mol Microbiol. 2000. 35:275–288.

25. Nolan LK, Wooley RE, Cooper RK. Transposon mutagenesis used to study the role of complement resistance in the virulence of an avian Escherichia coli isolate. Avian Dis. 1992. 36:398–402.

26. Olsén A, Jonsson A, Normark S. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature. 1989. 338:652–655.

27. Olsén A, Arnqvist A, Hammar M, Sukupolvi S, Normark S. The RpoS sigma factor relieves H-NS-mediated transcriptional repression of csgA, the subunit gene of fibronectin- binding curli in Escherichia coli. Mol Microbiol. 1993. 7:523–536.

28. Orndorff PE. Miller VL, Kaper JB, Portnoy DA, Isberg RR, editors. Escherichia coli type 1 pili. Molecular Genetics of Bacterial Pathogenesis. 1994. 1st ed. Washington DC: ASM Press;91–111.
29. Pourbakhsh SA, Boulianne M, Martineau-Doizé B, Dozois CM, Desautels C, Fairbrother JM. Dynamics of Escherichia coli infection in experimentally inoculated chickens. Avian Dis. 1997. 41:221–233.

30. Provence DL, Curtiss R III. Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect Immun. 1994. 62:1369–1380.

31. Rasmussen HN, Rasmussen OF, Andersen JK, Olsen JE. Specific detection of pathogenic Yersinia enterocolitica by two-step PCR using hot-start and DMSO. Mol Cell Probes. 1994. 8:99–108.

32. Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938. 27:493–497.
33. Runyan-Janecky LJ, Reeves SA, Gonzales EG, Payne SM. Contribution of the Shigella flexneri Sit, Iuc, and Feo iron acquisition systems to rion acquisition in vitro and in cultured cells. Infect Immun. 2003. 71:1919–1928.

34. Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: a Laboratory Manual. 1989. 2nd ed. New York: Cold Spring Harbor;1584.
35. Scaletsky ICA, Silva MLM, Trabulsi LR. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984. 45:534–536.

36. Schouler C, Koffmann F, Amory C, Leroy-Sétrin S, Moulin-Schouleur M. Genomic subtraction for the identification of putative new virulence factors of an avian pathogenic Escherichia coli strain of O2 serogroup. Microbiology. 2004. 150:2973–2984.

37. Schubert S, Rakin A, Karch H, Carniel E, Heesemann J. Prevalence of the "high-pathogenicity island" of Yersinia species among Escherichia coli strains that are pathogenic to human. Infect Immun. 1998. 66:480–485.

38. Sekizaki T, Nonomura I, Imada Y. Loss of virulence associated with plasmid curing of chicken pathogenic Escherichia coli. Nippon Juigaku Zasshi. 1989. 51:659–661.

39. Sperandio V, Silveira WD. Comparison between enterotoxigenic Escherichia coli strains expressing F42, F41 and K99 colonization factors. Microbiol Immunol. 1993. 37:869–875.

40. Stehling EG, Campos TA, Ferreira A, Silveira WD. Adhesion and invasion characteristics of a Septicaemic avian Escherichia coli strain are plasmid mediated. J Appl Res Vet Med. 2003. 1:27–36.
41. Szalo IM, Goffaux F, Pirson V, Piérard D, Ball H, Mainil J. Presence in bovine enteropathogenic (EPEC) and enterohaemorrhagic (EHEC) Escherichia coli of genes encoding for putative adhesins of human EHEC strains. Res Microbiol. 2002. 153:653–658.

42. Tarr CL, Large TM, Moeller CL, Lacher DW, Tarr PI, Acheson DW, Whittam TS. Molecular characterization of a serotype O121:H19 clone, a distinct Shiga toxin-producing clone of pathogenic Escherichia coli. Infect Immun. 2002. 70:6853–6859.

43. Taylor RK, Manoil C, Mekalanos JJ. Broad-host-range vectors for delivery of TnphoA: use in genetic analysis of secreted virulence determinants of Vibrio cholerae. J Bacteriol. 1989. 171:1870–1878.

44. Vidotto MC, Muller EE, de Freitas JC, Alfieri AA, Guimarães IG, Santos DS. Virulence factors of avian Escherichia coli. Avian Dis. 1990. 34:531–538.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download