Abstract
This study evaluated the effect of Matricaria chamomilla and vaccination frequency on cattle immunization against rabies. Four groups (n = 15 /group) were treated with or without Matricaria chamomilla CH12 and vaccinated with one or two doses of rabies vaccine (30 day interval). No effect of chamomile was found on cattle immunization against rabies; however, antibody titers were protective in cattle vaccinated twice, while 93.3% of cattle vaccinated only once had titers under 0.5 UI/ml after 60 days. In conclusion, the use of chamomile did not alter the humoral immune response in cattle, and two vaccine doses are suggested for achieving protective antibody titers.
Rabies is one of the most serious zoonoses in the world because it consists of fatal encephalitis that may be found in mammals, and occurs within a wide geographical range [1,4]. It is caused by a virus belonging to the genus Lyssavirus, of the family Rhabdoviridae [7,8], and is transmitted mainly by the hematophagous bat Desmodus rotundus in Latin America [1,8,10]. The most effective and inexpensive procedure for rabies control is the regular vaccination of cattle [1,7,11]. Albas et al. [1] and Lodmell et al. [9] showed that an adequate immune response was not achieved with a single rabies vaccination in some animals, although the vaccine producers stated that the antigenic levels per vaccine dose were within the normal range.
There is evidence to show that Matricaria chamomilla extract has immunomodulatory [2,5] and allogeneic properties on lymphocyte proliferation and activation of T cells, although further elucidation is needed [2]. The aim of this work was to evaluate the effect of Matricaria chamomilla CH12 as well as the number of doses of rabies vaccine, on the humoral immune response in cattle.
Sixty Nelore calves (Bos taurus indicus), about 12 months old, belonging to a farm situated in Lutecia, SP, Brazil, were studied. These calves were fed on Brachiaria decumbens from an extensive pasture system and supplemented with commercial mineral salt in an ad libitum regime. The Matricaria chamomilla CH12, was produced by the homeopathic Veterinary Laboratory (Arenales Fauna & Flora, Brazil). This product was composed of Matricaria chamomilla CH12 , milk CH12, Bixa orellana (0.75 g) and sucrose (100 g).
The experimental animals were randomly divided into four groups, FEV1, FEV2, V1, and V2 (15 animals per group). Cattle from FEV1 and FEV2 groups received Matricaria chamomilla CH12 mixed with mineral salt (Fosbov 15; Tortuga Cia Zootécnica Agrária, Brazil) for 90 days, and animals from groups V1 and V2 received only mineral salt. In the first 30 days, cattle were held for adaptation to pasture conditions and determination of the consumption of Matricaria chamomilla CH12 ingested with the mineral salt.
The determination of Matricaria chamomilla CH12 consumption per animal was performed in the first month of the experiment as follows: the mineral salt supplemented with Matricaria chamomilla CH12 was weighed, put in the feeder and, after 24 h, removed to be weighed again. The difference between the first and the second weighing divided by the number of animals that fed at the feeder was considered the average salt consumption per calf in 24 h. From these calculations, the amount of Matricaria chamomile consumed by each cattle was determined to be about 2 g a day.
We used a commercial liquid rabies vaccine (Rai-Vac; Fort Dodge Saúde Animal, Brazil) containing a suspension of fixed rabies Pasteur Virus cultured on baby hamster kidney (BHK)-21 cells, inactivated by beta-propiolactone, adsorbed to an adjuvant aluminum hydroxide and preserved with thimerosol at 1 : 10,000. The vaccine had antigen levels within the range recommended to reach an efficient immunological response, which was approved by the Brazilian Ministry of Agriculture, Livestock and Supply (MAPA). All the animals (groups FEV1, FEV2, V1 and V2) were vaccinated on day 0; the cattle from groups FEV2 and V2 received a second dose on day 30.
For blood sampling on days 0, 30 and 60, cattle were taken to the corral in the morning and restrained individually in a Brete chute. Blood (10 ml) was collected from the jugular vein in vacuum tubes with no anticoagulant. After the blood samples were clotted and centrifuged at 2,500 rpm for 10 min, the serum samples were stored at -20℃ for further determination of rabies-neutralizing antibodies in BHK-21 cells. The neutralizing antibodies were determined by serum neutralization in BHK-21 clone 13 cells. This test is based on the Rapid Fluorescent Focus Inhibition Test [13] and the Fluorescent Inhibition Microtest [14].
Analysis of variance followed by the Tukey-Kramer method was used to compare serum titers among the 4 groups on days 30 and 60 [3]. To compare the serum titers between days 30 and 60 within each group (groups FEV1, FEV2, V1 and V2), the Student t-test for paired samples was used. For all the analyses, the significance level was set at 5% [3].
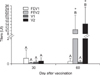
Rabies neutralizing antibody titers are typically used to evaluate the humoral immune response in cattle after rabies vaccination [1,8,11,12]. Moreover, it is recommended by the Centers for Disease Control and Prevention (USA). The first serum samples collected from all cattle tested on day 0 (FEV1, FEV2, V1 and V2) were not reactive for rabies, indicating that these animals had no prior contact with rabies virus or the vaccine. Thus, all the antibody titers found here were induced by the rabies vaccination during the study. In the present study, Matricaria chamomilla CH12 did not stimulate the production of rabies neutralizing antibodies (Fig. 1). On day 30 the antibody titers were similar between cattle that received Matricaria chamomilla CH12 and the respective treatment without supplementation (FEV1 × V1; FEV2 × V2). This suggested that the immunomodulatory effect of Matricaria chamomilla, found by Amirghofram et al. [2] and Gharagozloo and Ghaderi [5] in humans, did not occur in cattle for rabies immunization.
The World Health Organization recommends rabiesneutralizing antibody titers of at least 0.5 IU/ml for effective prevention in humans against rabies virus contamination. Some studies have stated that this neutralizing antibody titer is the minimal level required to protect cattle [1,6] against rabies. However, a descriptive analysis showed that 93.3% of the cattle that received a single vaccine dose (FEV1 and V1) had antibody titers under 0.5 IU/ml after 60 days of vaccination, independent of the treatment with Matricaria chamomilla CH12. This agrees with the report by Queiroz da Silva et al. [11], Albas et al. [1] showing that the humoral response induced by a single rabies vaccination is inefficient in protecting cattle against rabies virus because the antibodies are not produced in high quantities.
Still concerning imunization, cattle from groups FEV2 and V2, which were injected with rabies vaccine on days 0 and 30, had higher rabies-neutralizing antibody titers on day 60 compared to day 30 and to the groups vaccinated only once (Fig. 1). These animals had rabies-netralizing antibody titers above 0.5 UI/ml, i.e., 100% of the cattle were immunized against rabies on day 60. Indeed, other studies on cattle [1,11] show a significant increase in rabies-neutralizing antibodies after the second dose of rabies vaccine inactivated.
The results found in the present study lead to the conclusion that the use of Matricaria chamomilla CH12 added to mineral salt did not affect the humoral immune response. In addition, two doses of rabies vaccine were shown to be required for rabies protection (≥ 0.5 UI/ml) in cattle.
Figures and Tables
Fig. 1
Mean rabies-neutralizing antibody titers from Nelore cattle on days 30 and 60. Cattle from groups FEV1 and FEV2 received Matricaria chamomilla CH12 mixed with the mineral salt and were vaccinated with one and two doses of rabies vaccine, respectively. Cattle from groups V1 and V2 had only mineral salt and one and two doses of rabies vaccine, respectively. An asterisk (*) indicates significant statistical difference between observation days 30 and 60 within each group. In 30 days, letter A means statistical non-significant among all groups. In 60 days, letter A and B means statistical significant between vaccination and non-vaccination all group, respectively.
Acknowledgments
The author would like to thank the "Homeopatia Arenales Fauna e Flora", Presidente Prudente, SP, Brazil.
References
1. Albas A, Pardo PE, Bremer-Neto H, Gallina NMF, Mourão Fuches RM, Sartori A. Vacinação anti-rábica em bovinos: comparação de cinco esquemas vacinais. Arq Inst Biol. 2005. 72:153–159.
2. Amirghofran Z, Azadbakht M, Karimi MH. Evaluation of the immunomodulatory effects of five herbal plants. J Ethnopharmacol. 2000. 72:167–172.

3. Banzatto DA, Kronka SN. Experimentação Agrícola. 1995. 3rd ed. Jaboticabal: Funep;247.
4. Coleman PG, Fèvre EM, Cleaveland S. Estimating the public health impact of rabies. Emerg Infect Dis. 2004. 10:140–142.

5. Gharagozloo M, Ghaderi A. Immunomodulatory effect of concentrated lime juice extract on activated human mononuclear cells. J Ethnopharmacol. 2001. 77:85–90.

6. Giometti J, Chiacchio SB, Albas A, Pardo PE, Bremer-Neto H, Giometti AI, Reis LSLS. Influência da suplementação com crômio na resposta imune humoral anti-rábica em Bovinos. Arq Inst Biol. 2006. 73:421–427.

7. Hankins DG, Rosekrans JA. Overview, prevention, and treatment of rabies. Mayo Clin Proc. 2004. 79:671–676.

8. Kotait I, Gonçalves CA, Peres NF, Souza MCAM, Targueta MC. . Controle da Raiva dos Herbívoros. Manual Técnico 1. 1998. São Paulo: Instituto Pasteur;1–11.
9. Lodmell DL, Smith JS, Esposito JJ, Ewalt LC. Cross-protection of mice against a global spectrum of rabies virus variants. J Virol. 1995. 69:4957–4962.

10. Piza AT, Pieri KMS, Lusa GM, Caporale GMM, Terreran MT, Machado LA, Zanetti CR. Effect of the contents and form of rabies glycoprotein on the potency of rabies vaccination in cattle. Mem Inst Oswaldo Cruz. 2002. 97:265–268.

11. Queiroz da Silva LH, Cardoso TC, Perri SHV, Pinheiro DM, Carvalho C. Pesquisa de anticorpos anti-rábicos em bovinos vacinados da região de Araçatuba, SP. Arq Inst Biol. 2003. 70:407–413.
12. Sihvonen L, Kulonen K, Neuvonen E. Immunization of cattle against rabies using inactivated cell culture vaccines. Acta Vet Scand. 1994. 35:371–376.

13. Smith JS, Yager PA, Baer GM. Meslin FX, Kaplan MM, Koprowski H, editors. A rapid fluorescent focus inhibition test (RFFIT) for determing rabies virus-neutralizing antibody. Laboratory Techniques in Rabies. 1996. 4th ed. Geneva: World Health Organization;181–192.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download