Abstract
Renal length, height, width, resistive index (RI), size of cortex, and medulla were determined by renal ultrasonography in 50 healthy Korean domestic short-hair cats. In the sagittal plane, the renal length was 3.83 ± 0.51 cm (mean ± SD) in the left kidney and 3.96 ± 0.48 cm in the right kidney, whereas the renal height was 2.42 ± 0.27 cm in the left kidney and 2.36 ± 0.28 cm in the right kidney. In the transverse plane, the renal height was 2.42 ± 0.28 cm in the left kidney and 2.38 ± 0.27 cm in the right kidney, whereas the renal width was: 2.65 ± 0.35 cm in the left kidney and 2.63 ± 0.31 cm in the right kidney. In the dorsal plane, the renal length was 3.84 ± 0.53 cm in the left kidney and 3.97 ± 0.54 cm in the right kidney, whereas the renal width was 2.65 ± 0.34 cm in the left kidney and 2.66 ± 0.33 cm in the right kidney. There were no significant differences (p > 0.05) among the same structure sizes measured in different planes. In the sagittal plane, the size of the renal cortex was 0.47 ± 0.08 cm in the left kidney and 0.47 ± 0.08 cm in the right kidney, whereas of the size of the renal medulla was 0.55 ± 0.30 cm in the left kidney and 0.50 ± 0.07 cm in the right kidney. RI evaluated by pulsed wave Doppler sonography was 0.52 ± 0.05 in the left kidney and 0.55 ± 0.05 in the right kidney. The actual renal dimensions determined by gross examination were not statistically different from those determined by ultrasonography. Furthermore the renal dimensions and RI were statistically correlated to the body weight of cats.
Ultrasonography is widely applied to detect the presence of abnormal structures and morphological changes in solid organs and is useful to narrow down the differential diagnosis [6,13]. Ultrasonographic evaluation is especially useful for assessing kidneys because important anatomic information concerning the size, shape, and internal architecture can be obtained even in the presence of impaired renal function or abdominal fluid [1]. Compared to conventional survey radiology, ultrasonography can better visualize kidneys in emaciated animals and those with retroperitoneal fluid. Subcapsular fluid, localized perirenal fluid, small renal or perirenal masses, and pelvic or ureteral dilation can be also easily detected in ultrasonography. Ultrasound-guided interventional procedures and Doppler ultrasound imaging enable us to better assess renal functional status [7].
Reduced renal artery diastolic flow indicates a generalized increase in renal vascular resistance. Although this finding is nonspecific, it is a good indicator for acute renal disease, acute tubular necrosis, and renal obstruction [11]. To determine this renal vascular resistance, in practice, resistive index (RI) as determined by Doppler ultrasonography is widely applied. Signals from the arteries near the renal hilum (segmental or interlobar) and corticomedullary junction (arcuate) are used for the RI determination. Because of the inaccessibility to these small vessels, the frequency shift is used to provide a relative assessment of blood flow velocity during systole and diastole [12]. Because the normal reference range of RI is less than 0.70, any increased RI value suggests that the increased renal vascular resistance is due to certain renal disease.
Although a few studies have been already reported about normal renal dimensions and RI, the comparison between the actual and ultrasonographic dimension is rarely reported [4,5]. Also most of the cat populations enrolled in previous studies included a wide range of cat breeds. Therefore in this study, we restricted our research to a single breed of cat (domestic short-hair cat) and evaluated the renal dimensions and RI in a clinically healthy cat population. Furthermore to provide better reference index, we compared the actual renal dimensions determined by necropsy to those determined by ultrasonography.
Fifty healthy adult cats (23 females and 27 males), weighing 2.1~5.5 kg were used. The cats were considered healthy on the basis of physical examination and normal CBC, serum urea nitrogen concentration, and routine urinalysis. The health status of the cats were re-evaluated 2 weeks after the initial evaluation. Only healthy cats were enrolled in this study. Our study was approved by the Animal Ethics Committee of Kangwon National University and was performed under strict adherence to the guidelines which included animal care, euthanasia, and disposal of dead animals.
For each cat, preparations for an ultrasound scan included a 12-h fast, the availability of water at all times, and a tepid water enema 1 to 2 h before the procedure. The ventral abdominal hair coat was clipped from the costal arch to the iliac wings. The cat was anesthetized by atropine (0.03 mg/kg, SC), ketamine (10 mg/kg, IM) and xylazine (1 mg/kg, IM).
Cats were placed in dorsal recumbency for survey ultrasonography. A water-soluble coupling gel was applied liberally to the ventral abdomen to permit sound conduction. Ultrasound scans were performed, using a static B-mode articulated scan arm and a 4~9 MHz transducer (Sonoace 8000SE; Medison, Korea). The longitudinal axis of the left kidney was located by use of a survey scan. Sagittal scans were begun at the medial margin of the kidney, and a serial sequence of sagittal scans was made at 0.5-cm intervals until the most lateral margin of the kidney was no longer visible (Fig. 1). The scan arm was then rotated 90°. Cross sectional (transverse) images were obtained, beginning at the cranial pole of the left kidney, and a serial sequence of transverse scans was made at 0.5-cm steps until the caudal pole was no longer visible (Fig. 2). Dorsal scans were made as described in sagittal scans after the scan probe move laterally (Fig. 3). The procedure was repeated for the right kidney.
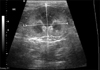
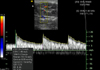
To obtain the RI, a renal interlobar or arcuate artery was identified with color Doppler. The Doppler tracing was then obtained and recorded by placing a gate of 2.5 mm width (adjusted when necessary) over the artery, setting the wall filter to 125 Hz, and selecting the smallest scale that displayed the flow without aliasing (Fig. 4). In most cats, one five-second waveform strip from one artery for each kidney was recorded. The peak systolic and end diastolic velocities were measured by the methods used in a previous report [4].
To measure the actual dimensions of the kidneys, twenty-seven of 50 cats were necropsied after ultrasonographic examination. Both kidneys were removed and measured using Vernier calipers (Mitutoyo, Japan).
The correlation coefficiency for the group means for body weight and renal dimension were calculated and compared using statistical software packages (SAS Ver 8.2; SAS Institute, USA). Renal dimensions obtained through each different ultrasonographic plane and gross measurements (necropsy) were compared using paired t-test.
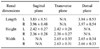
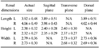
Renal dimensions measured by ultrasonography in 50 domestic shorthair cats including renal length, height, and width were summarized in Table 1. No statistically significant difference between the renal dimensions measured in different songraphic angles was observed (p > 0.05). The mean thicknesses of renal cortex and medulla were 0.47 ± 0.08 cm (mean ± SD) in the left kidney and 0.47 ± 0.08 cm in the right kidney, and 0.55 ± 0.30 cm in the left kidney and 0.50 ± 0.07 cm in the right kidney, respectively. The means of RI of both kidneys were 0.52 ± 0.05 in the left kidney and 0.55 ± 0.05 in the right kidney.
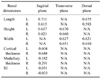
Renal dimensions measured by gross examination in 27 domestic shorthair cats including renal length, height, and width were summarized in Table 2. No statistically significant difference between the renal dimensions as measured by two different measurements was observed (p > 0.05).
Since the cats enrolled in this study had a wide range of body weight (2.1~5.5 kg), the measured renal dimensions were statistically analyzed to identify the correlation index (Table 3). Although a high degree of correlation to body weight has been observed in the renal dimensions (renal length, height, and width) and the renal cortical thickness, a low degree of correlation has been observed in the renal medullary thickness and RI (Table 3).
Normal echocardiographic structure of the kidney is influenced by the echocardiographic angle (plane), breathing patterns of animals, degree of interference by other organs (e.g. liver and spleen), and the skills of the practitioner, although the high-quality ultrasound machine and appropriate transducer were used in the examinations. In addition, the slight pressure on abdomen by transducer can displace the location and orientation of the kidney. However it can not be avoided, since feline kidneys are extremely mobile. In the dorsal plane, the correct measurement of renal width is problematic, because of the abundance of connective tissues in the renal hillus. This can be overcome in the cross measurement by using the transverse plane. Generally the caudal and cranial pole of kidney is sometimes unclear. These can be better visualized by using the dorsal plane. As in previous literature which had mentioned the problems encountered in the measurement of renal dimensions, we measured renal dimensions at the three different angles and measured three times in each case to minimize factors affecting correct measurement in this study.
The renal dimensions that we measured in this study were similar to previous reports from those measured by others [14], because the weight ranges of the cats were not significantly different. Furthermore the discrepancy of the renal dimensions measured by gross (necropsy) and ultrasonographic examination was statistically insignificant. However, the renal cortical thickness was not similar to previous reports of the measurement by others [14], although the medullary thickness was not different. However, in the study by others [14], cortical dimensions were slightly greater because their measurement included the bright diverticular echoes, which could contribute to the discrepancy from our results. However, because the cortical and medullary dimensions were different even in the same cats depending on the anatomical location measured and the dimensions of the renal medulla were not able to be clearly defined due to the unclear borders of renal sinuses in the transverse plane, the clinical application of renal cortical and medullary dimensions is limited.
Because renal function is dependent on renal blood flow, glomerular and tubular function, and urine flow, the measurement for renal blood flow (e.g. RI) may help for the diagnosis, treatment, and prognosis of renal disease. Renal arterial RI is the ratio of systolic to diastolic velocity and is used to estimate vascular resistance. Since increased vascular resistance decreases diastolic velocity, the increased renal arterial RI implies reduced renal blood flow. A significant relationship between RI and acute renal failure has been reported in veterinary literature [4]. However, in humans, the reliability of renal arterial RI measurements is controversial [2,3], although it has proven to be useful in humans for evaluating renal transplant complications [10]. Because RI is influenced by age, the patency of urinary tract, and the animal's circulatory status and because the normal reference range of RI in cats are too wide, clinical application of RI still limited, although one study reported a high specificity of RI for canine renal diseases [4]. As noticed in previous studies [4,5], the range of RI in healthy cats was wide (left: 0.42~0.71, right: 0.41~0.73). The means of RI in both kidneys were similar to previous reports [9], although the different anesthetic protocol was used in this study. One study found RI was not markedly influenced by deep sedation, as reported previously [8]. However, this study used a different anesthetic protocol, which might potentially affect RI in our study population, although the means of RI in both kidneys were similar to others [9]. Probably, the actual RI in our study population might be higher than others [9], since it might be underestimated due to influence from the hypotensive effect from xylazine (used in this study). Otherwise, xylazine might minimally influence the RI in our study population, so that the mean RI was similar to others [9]. Because we did not clarify this issue prior to study, the mean RI found in this study might be different from the RI in cats without chemical restraints. Therefore, future studies should be directed to clarify the effects on RI from different type of chemical restraints.
Although the cats enrolled in this study have a wide range of body weights, the measured renal dimensions except renal medullary thickness were statistically closely correlated. Probably this result was because there were no severely obese and emaciated cats included in this study.
In summary, renal dimensions and RI measured by ultrasonography in 50 Korean cats were similar to those measured by others and gross examinations. The renal dimensions and RI were statistically correlated to the body weight of cats.
Figures and Tables
Fig. 1
Determination of renal length and height on the sagittal plane. When two bright parallel bars formed by cross sectioned pelvic diverticulum were clearly visible, the renal length and height were measured. L: length, H: height, C: cortex, M: medulla.
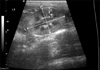
Fig. 2
Determination of renal length and height on the transverse plane. When the "C"-sign of the renal crest was clearly visible, the renal length and height were measured. H: height, W: width.
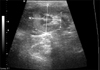
Table 1
Comparison of renal dimensions of 50 Korean domestic short-hair cats measured in different ultrasonographic angles
Acknowledgments
This study was supported from Institute of Veterinary Science, Kangwon National University.
References
1. Armbrust LJ, Biller DS, Hoskinson JJ, Meier HT, Lora-Michiels M. The basics of renal ultrasonography. Vet Med. 2001. 96:114–133.
2. Genkins SM, Sanfilippo FP, Carroll BA. Duplex Doppler sonography of renal transplants: lack of sensitivity and specificity in establishing pathologic diagnosis. Am J Roentgenol. 1989. 152:535–539.

3. Jurriaans E, Dubbins PA. Renal transplantation: the normal morphological and Doppler ultrasound examination. J Clin Ultrasound. 1992. 20:495–506.

4. Morrow KL, Salman MD, Lappin MR, Wrigley R. Comparison of the resistive index to clinical parameters in dogs with renal disease. Vet Radiol Ultrasound. 1996. 37:193–199.

5. Nyland TG, Fisher PE, Doverspike M, Hornof WJ, Olander HJ. Diagnosis of urinary tract obstruction in dogs using duplex Doppler ultrasonography. Vet Radiol Ultrasound. 1993. 34:348–352.

6. Osborne CA, Finco DR. Canine and Feline Nephrology and Urology. 1995. 8th ed. Baltimore: Williams & Wilkins;370–464.
7. Platt JF. Duplex Doppler evaluation of native kidney dysfunction: obstructive and nonobstructive disease. AJR Am J Roentgenol. 1992. 158:1035–1042.

8. Pollard R, Nyland TG, Bernsteen L, Gregory CR, Hornof WJ. Ultrasonographic evaluation of renal autografts in normal cats. Vet Radiol Ultrasound. 1999. 40:380–385.

9. Quarto di Palo F, Rivolta R, Elli A, Castagnone D. The well-functioning renal graft evaluated by color Doppler flowmetry. Nephron. 1995. 70:314–318.

10. Rifkin MD, Needleman L, Pasto ME, Kurtz AB, Foy PM, McGlynn E, Canino C, Baltarowich OH, Pennell RG, Goldberg BB. Evaluation of renal transplant rejection by duplex Doppler examination: value of the resistive index. AJR Am J Roentgenol. 1987. 148:759–762.

11. Rivers BJ, Walter PA, Letourneau JG, Finlay DE, Ritenour ER, King VL, O'Brien TD, Polzin DJ. Duplex Doppler estimation of resistive index in arcuate arteries of sedated, normal female dogs: implications for use in the diagnosis of renal failure. J Am Anim Hosp Assoc. 1997. 33:69–76.

12. Rivers BJ, Walter PA, O'Brien TD, Polzin DJ. Duplex Doppler estimation of Pourcelot resistive index in arcuate arteries of sedated normal cats. J Vet Intern Med. 1996. 10:28–33.

13. Walter PA, Feeney DA, Johnston GR, Fletcher TF. Feline renal ultrasonography: quantitative analyses of imaged anatomy. Am J Vet Res. 1987. 48:596–599.
14. Walter PA, Johnston GR, Feeney DA, O'Brien TD. Renal ultrasonography in healthy cats. Am J Vet Res. 1987. 48:600–607.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download