Abstract
Due to the therapeutic potential of gene therapy for neuronal injury, many studies of neurotrophic factors, vectors, and animal models have been performed. The presumed dog β-nerve growth factor (pdβ-NGF) was generated and cloned and its expression was confirmed in CHO cells. The recombinant pdβ-NGF protein reacted with a human β-NGF antibody and showed bioactivity in PC12 cells. The pdβ-NGF was shown to have similar bioactivity to the dog β-NGF. The recombinant pdβ-NGF plasmid was administrated into the intrathecal space in the gene therapy group. Twenty-four hours after the vector inoculation, the gene therapy group and the positive control group were intoxicated with excess pyridoxine for seven days. Each morning throughout the test period, the dogs' body weight was taken and postural reaction assessments were made. Electrophysiological recordings were performed twice, once before the experiment and once after the test period. After the experimental period, histological analysis was performed. Dogs in the gene therapy group had no weight change and were normal in postural reaction assessments. Electrophysiological recordings were also normal for the gene therapy group. Histological analysis showed that neither the axons nor the myelin of the dorsal funiculus of L4 were severely damaged in the gene therapy group. In addition, the dorsal root ganglia of L4 and the peripheral nerves (sciatic nerve) did not experience severe degenerative changes in the gene therapy group. This study is the first to show the protective effect of NGF gene therapy in a dog model.
There are many studies on the treatment of neuronal injuries. Among them, gene therapy has the potential to be important in pathological responses to injury and to the enhancement of functional recovery [1,5,8,20,21].
For gene therapy of neuronal injury, various neurotrophic factors, vectors, and animal models need to be considered. Most of the previous studies on neuroprotective gene transfer used genetically engineered virus vectors, such as the herpes-simplex type I virus and the adenovirus [6-8,17,20]. Among the various neuropathies of the nervous system, peripheral neuropathies are characterized by motor, sensory, and sympathetic deficits. Sensory neuropathies are frequently associated with diabetes, anticancer therapies, and metabolic disorders [4,9]. Various drugs have been used, such as cisplatin, taxol, and acrylamide for the induction of sensory neuropathies [4,14]. There are many neurotrophic factors for gene therapies, such as nerve growth factor (NGF), and the brain-derived neurotrophic factor, neurotrophin-3 [11]. Nerve growth factor is one of the growth factors now being recognized as essential to the survival and maturation of sensory and sympathetic neurons, along with other neurotrophins [16]. NGF consists of three subunits, α, β, and γ, and forms a 7S complex of approximately 27 kDa. This complex contains two identical 118 amino acid β chains that are solely responsible for the trophic activity of NGF [19]. Although a specific role for β-NGF in the adult peripheral nervous system has not been established, there are many studies concerning the effect of β-NGF in animal models of peripheral neuropathy, and these studies have shown that β-NGF had protective effects from the degeneration characteristic of peripheral neuropathy in sensory neurons [3,10].
In humans, there are many neurodegenerative disorders and there have been many trials to treat these disorders with neurotrophic factors [11]. In the veterinary field, many neurodegenerative disorders also exist, and more trials should be done to identify and characterize appropriate treatments. In some animals, species-specific β-NGF sequences, which are needed for clinical trials, have already been determined [2,19]. To our knowledge, this is the first study about the presumed function of dog β-NGF (pdβ-NGF) in vitro and in vivo.
The ultimate goal of this study was to determine the effect of the cytomegalovirus (CMV) vector-mediated gene transfer of the pdβ-NGF in vitro and gene therapy using recombinant pdβ-NGF plasmid in the dog model, having pyridoxine-induced peripheral neuropathy.
Genomic DNA was extracted from the tonsil tissue of a healthy adult male mongrel dog using the DNeasy Tissue Kit (Qiagen, Germany). Primers were generated using the sequences of human and mouse β-NGF genes to amplify partial dβ-NGF sequences. PCR using the primers NGF-1F (5'-TCAGCATTCCCTTGACACWG-3') and NGF-1R (5'-AGCCTTCCTGCTGAGCAC-3') was performed for 35 cycles at 94℃ for 1 min, 44℃ for 1min and 72℃ for 1 min (Fig. 1). PCR using the primers NGF-2F (5'-AGTTCTCGGTGTGCGACAG-3') and NGF-2R (5'-GCCCAGGAGAGTGTGGAG-3') was also performed for 35 cycles at 94℃ for 1 min, 55℃ for 1 min and 72℃ for 1 min (Fig. 1). A PCR product of approximately 600 bp was expected when using the primers NGF-1F and NGF-1R and 400 bp with the primers NGF-2F and primer NGF-2R. Overlapping PCR was performed to combine the PCR products of the partial dβ-NGF using NGF-1F and NGF-2R. This overlap PCR was performed for 35 cycles at 94℃ for 1 min, 58℃ for 1 min and 72℃ for 1 min. The size of this PCR product was confirmed to be approximately 660 bp (pcDNA1) using 1.5% agarose gel electrophoresis. The cloning of pcDNA1 was performed with pCR2.1 vector (Invitrogen, USA) and plasmid DNA was extracted from Escherichia coli TOP10 cells (Invitrogen, USA) with the plasmid purification kit (NucleoGen Biotechnology, Korea). The plasmids were sequenced by Takara-Korea Biomedical Inc. The remainder of dβ-NGF DNA was synthesized artificially based on the sequence of the predicted Canis familiaris nerve growth factor beta (5'-ATGTCCATGTTGTTCTACACTCTGATCACAGCTCTTCTGATCGGCATCCGGGCAGAACCGCATCCAGAGAGCCATGTCCCAGCAGGACACGCCATCCCCCACGCCCACTGGACTAAGCTTCAGCATTCCCTT-3'; GeneBank sequence entry XM_540250; NCBI, USA) (Fig. 1). Overlapping PCR was performed to combine the synthesized oligonucleotide and pcDNA1. The sense primer (Bgl2 + NGF-F) with a sequence of 5'-GGCAGATCTATGTCCATGTTG-3', and the antisense primer (EcoR1 + NGF-R) with a sequence of 5'-GGAGAATTCTCAGGCTCGTCT-3', were used for the overlap PCR. The sense primer had the BglII (TaKaRa Bio, Japan) restriction site and the antisense had the EcoR I (TaKaRa Bio, Japan) restriction site. PCR was performed for 35 cycles at 94℃ for 1 min, 53℃ for 1 min and 72℃ for 1 min. The obtained PCR product was expected to be 725 bp (pcDNA2). The pcDNA2 was cloned and the nucleotide sequence was analyzed. The pcDNA2 clone was named the presumed dog β-NGF (pdβ-NGF).
The CMV vector (phCMV1; Gene Therapy Systems, USA) was chosen to construct the recombinant pdβ-NGF (rpdβ-NGF) plasmid. The phCMV vector and pcDNA2 were digested with Bgl II and EcoR I restriction enzymes, and purified following separation on a 1.5% agarose gel. Ligation of the purified phCMV1 and pcDNA2 was performed using T4 DNA ligase (TaKaRa Bio, Japan). The rpdβ-NGF plasmid was transformed into Escherichia coli TOP10 cells and DNA extracted with a plasmid purification kit.
The rpdβ-NGF plasmid and a separate phCMV1 vector plasmid prepared for transfection were free of protein, RNA and chemical contamination (A260/A280 ratio of 1.9) and had a final concentration of 0.4 mg/ml.
A cationic liposome-mediated transfection technique (Gene therapy systems, USA) was carried out to deliver the two plasmids into Chinese hamster ovary (CHO) cells (Korean Cell Line Bank, Korea). Four days after the transfections, supernatants were collected and filtered through a 0.22-µm filter (Millipore, USA).
pdβ-NGF protein levels were measured using the Duoset Enzyme-Linked Immunosorbent Assay (ELISA) development system (R&D Systems, USA). This system used a sandwich ELISA method with anti-human β-NGF as the detection antibody and was performed according to the manufacturer's recommendations. The bioactivity of the pdβ-NGF protein was assessed using the rat pheochromocytoma cell line (PC12 cell; Seoul National University, Korea). PC12 cells were plated at a density of 5 × 104 cells/ml in 24-well tissue culture plates (Falcon, USA). One ml of supernatant from the rpdβ-NGF-tranfected CHO cells was added to the PC12 cell cultures. Supernatant from CMV-transfected CHO cells, was used as a negative control. PC 12 cells were monitored daily by microscopic examination.
Ten mongrel dogs (5 males and 5 females) roughly 2 years of age were used in this experiment. The body weights ranged from 4 to 6 kg. Among them, two dogs were in the negative control group, four dogs were in positive control group and four dogs were in the experimental group with gene therapy. All of the dogs were clinically judged to be in good health and neurologically normal, and had their own admission number from the Institute of Laboratory Animal Resources, Seoul National University (SNU-060623-1). During the experiment, all of the dogs were cared for according to the Animal Care and Use Guidelines (Institute of Laboratory Animal Resources, Seoul National University, Korea). Body weights of test dogs were measured every morning during the test period.
The CMV vector containing rpdβ-NGF was prepared in advance. The cationic polymer transfection reagent (Polyplus transfection, France) was used to transport these plasmids into the intrathecal region. Each plasmid was condensed with in vivo-jetPET-Gal reagent at a 10-N/P ratio (measure of the ionic balance of the complexes). First, the prepared plasmids were diluted with 200 µl of 5% glucose (w/v) and an appropriate amount of in vivo-jetPET-Gal reagent in 200 µl of 5% glucose (w/v). Second, 200 µl of in vivo-jetPET-Gal solution was added to the plasmid solution followed by incubation for 15 min at room temperature. Third, the mixture was injected to the dogs of the gene therapy group (n = 4) through intrathecal injection using a 27-gauge needle. Before this administration, the dogs of the gene therapy group were anesthetized with zoletil.
Twenty-four hours after vector inoculation, the dogs from the gene therapy group (n = 4) and the positive control group (n = 4) were intoxicated with pyridoxine (Sigma, France). The pyridoxine was prepared in distilled water (100 mg/ml) immediately before injection, and administered at 150 mg/kg subcutaneously once a day in the morning, for 7 days. Dogs in the negative control group (n = 4) received vehicle (isoosmotic sterile aqueous solution of sodium chloride).
Postural reaction (wheelbarrowing, hopping, extensor postural thrust, placing, tonic neck reaction and proprioceptive positioning) assessments were done on all dogs every morning during the test period.
All of the dogs were preanesthetized with atropine (0.1 mg/kg of body weight, IM). Anesthesia was induced with diazepam and was maintained with isoflurane through a semiclosed system. Subcutaneous temperature was maintained at 37~38℃. Neuropack2 (Nihon Kohden, Japan) was used for all recordings. All measurements were performed in the left hindlimb. M wave was recorded for the tibial nerve, using 1 Hz, 0.5 ms, supramaximal stimulus. Stimulating electrodes were positioned in the distal tibial nerve. The recording electrode was positioned in the plantar interosseous muscle. The ground electrode was positioned between the stimulating electrode and the recording electrode. The recording electrode was a bipolar needle electrode. The Hoffman (H)-reflex was recorded using 1 Hz, 0.5 ms, submaximal stimulus. The stimulating electrode was positioned in the tibial nerve adjacent to the hook and the recording and ground electrodes were positioned in the same site of the tibial nerve where the M wave was measured. All measurements were performed at least eight times. Electrophysiological recordings were performed twice, once before the experiment and once after the test period.
After the experimental period (10 days from the start of the experiment), the dogs were anesthetized with a high dose of Tiletamine/zolazepam and propofol, and perfused transcardially with 0.1 M phosphate-buffered saline (PBS), followed by 4% paraformaldehyde in 0.1 M PBS to induce euthanasia. After perfusion, tissues (lumbar spinal cord (L4), left and right dorsal root ganglia of L4 and sciatic nerve) were quickly removed and post-fixed for 4~6 h in the same fixative at 4℃ and embedded in paraffin. The tissues were sectioned serially with a thickness of 5 µm using a microtome (Reichert-Jung, Germany) and floated onto gelatine-coated slides. Next, they were deparaffinized in xylene, rehydrated in a descending ethanol series, and stained with hematoxylin and eosin. The sections were observed using an Olympus BX51 microscope (Olympus, Japan) attached to a IMT2000 digital camera (iMTechnology, Korea) and images were captured using Adobe Photoshop version 6.0 software via IMT2000.
The results of nucleotide sequence analysis showed that the gene cloned in pcDNA1 had a high degree of sequence homology with other mammalian β-NGF genes. The pcDNA1 sequence shared 86% and 83% sequence homology with that of human (GeneBank sequence entry NM_002506; NCBI, USA) and mouse (GeneBank sequence entry NM_013609; NCBI, USA) β-NGF sequences, respectively. The remaining part of dβ-NGF DNA, which was synthesized artificially, was included in the 5' region of the dβ-NGF open reading frame (ORF) and contained 132 base pairs. Overlapping PCR was performed to combine the synthesized oligonucleotide and pcDNA1, and the obtained PCR product was 725 bp (pcDNA2). Again, the nucleotide sequence analysis showed that the cloned gene had a high degree of sequence homology with other mammalian β-NGF genes. The pcDNA2 clone was named the presumed dog β-NGF (pdβ-NGF) (Fig. 1). With the additional sequence contributed by the syntesized oligoneucleotide, the shared homology changed to 85% and 81% compared to human (GeneBank sequence entry NM_002506; NCBI, USA) and mouse (GeneBank sequence entry NM_013609; NCBI, USA) β-NGF sequences, respectively. The deduced pdβ-NGF amino acid sequence shared 90% and 82% homology with that of human (GeneBank sequence entry NM_002506; NCBI, USA) and mouse (GeneBank sequence entry NM_013609; NCBI, USA), respectively.
Four days after transfection to CHO cells, pdβ-NGF protein was obtained from the supernatant. The filtered supernatant was measured with sandwich ELISA of human β-NGF. The results indicated that 53 pg/ml of pdβ-NGF protein existed in the supernatant. The bioactivity of pdβ-NGF protein was assessed using the rat pheochromocytoma cell line (PC12 cell; Seoul National University, Korea). Seven days after treatment with filtered supernatant, a small number of PC12 cells had neurite outgrowth, while the PC12 cells in the negative control group maintained their original morphology (Fig. 2).
The weight measurements showed that there was weight loss only in the positive control group. There were no weight changes in the negative control group or the gene therapy group. The difference in body weight of the positive control group was statistically significant (p < 0.05). The differences in body weight of the negative control group and the gene therapy group were not statistically significant (p < 0.05).
All the dogs in the positive control group developed a neurological disorder, characterized by ataxia involving first, and most prominently, the hindquarters. All of the dogs in the positive control group started to show proprioceptive abnormalities involving the hindquarters as detected by the postural reaction test (wheelbarrowing, hopping, extensor postural thrust, placing, tonic neck reaction and proprioceptive positioning) on the third day of pyridoxine injection. On the fourth day of pyridoxine injection, all dogs held their hindlimb stiffly when standing. These conditions were maintained until the end of the pyridoxine injection. On the other hand, all of the dogs in the negative control group and the gene therapy group were normal during the postural reaction test.
Electrophysiological readings were recorded to measure M wave and H reflex in all treatment groups. The M wave amplitude of all the dogs in the negative control group, the positive control group, and the gene therapy group showed no remarkable change before and after the pyridoxine administration as confirmed by statistical analysis (p < 0.05). However, there was a remarkable change in H reflex before and after the pyridoxine intoxication in the positive control group. Before the pyridoxine intoxication, the amplitude of H reflex was 0.52 ± 0.06 mV. After the pyridoxine intoxication, however, there was no consistently detectable H reflex in the positive control group. The H reflexes in the negative control group and the gene therapy group did not change before and after the pyridoxine intoxication as confirmed by statistical analysis (p < 0.05).
Histopathologically, there were no lesions in the lateral, dorsal or ventral funiculus, or in the gray matter of L4 in the negative control group (Fig. 3A). The axons and myelin was disrupted with vacuolation in the positive control group (Fig. 3B). In the gene therapy group, swollen axons were occasionally seen in the dorsal funiculi of L4 (Fig. 3C).
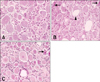
There were no lesions in the dorsal root ganglia (DRG) of L4 in the negative control group (Fig. 4A). However, severe chromatolysis was observed in the neurons of DRG of L4 in the positive control group. Vacuolation was also observed in the neurons. Occasionally, some neurons were necrotic, and were characterized by pyknotic nuclei and eosinophilic cytoplasm (Fig. 4B). Some neurons had pyknotic nuclei and eosinophilic cytoplasm in the gene therapy group (Fig. 4C).
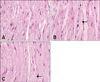
There were no lesions in the axons or myelin in peripheral nerves (sciatic nerve) of the negative control group (Fig. 5A). Severe vacuolation was seen in the myelin in peripheral nerves (sciatic nerve) of the positive control group (Fig. 5B). There was mild vacuolation in the myelin in peripheral nerves (sciatic nerve) of the gene therapy group (Fig. 5C).
Recently, significant efforts have been made to develop gene therapies in the neurologic area. For the development of gene therapies, the selection of appropriate growth factors, vectors, delivery reagents, animal models, and administration route are important.
To our knowledge, this is the first study of dog β-NGF. In this study, we were not able to clone the full-length ORF of dβ-NGF, only a partial region. We believe this is because NGF contents in dog tissues are low. To compensate for this, the remaining portion of the dβ-NGF ORF was synthesized artificially. To generate a functional NGF protein, it is very important that the correct tertiary structure is formed. For this reason, the CHO cell expression system was chosen instead of the E. coli expression system [4]. Since the amount of secreted proteins was small in the CHO cell expression system, only small amounts of recombinant proteins were obtained. The ELISA showed that 53 pg/ml of pdβ-NGF protein existed in the supernatant. This amount (53 pg/ml) by itself may not bear meaning at this stage since we do not know the exact ELISA cross-reactivity ratio between human and dog β-NGF. However, the findings are significant in that they indicate for the first time that the presumed dog's recombinant proteins were reactive to the anitibody used in the human ELISA kit used in this study. Based on these data, it is suggested that pdβ-NGF DNA has the equivalent bioactivity of the dog β-NGF. Since many neurodegenerative disorders also exist in the veterinary field, there should be more trials to analyze the pathogenesis and to develop appropriate treatments. These clinical investigations require dog-specific β-NGF. The results obtained in this study will open the way for basic and applied research on dog β-NGF as a neurotrophic factor.
For gene transfection in an animal model, several kinds of vectors and transfection agents were used. Although some viral vectors may be efficient in transducing cells, they are also associated with higher biological risks. Compared with viral vectors, a CMV vector is very safe when used with animal models. Cationic liposomes, which condense and introduce DNA into cells, have been considered to be more suitable candidates for gene therapy due to their non-immunogenicity, non-toxicity, and relative biological safety [20].
Studies on the treatments of nervous system diseases are very difficult because of the blood-brain barrier (BBB). The plasmid DNAs are not small enough to penetrate the BBB. Therefore, systemic injections of plasmid DNAs could not performed. Direct intratheral injection into the cisterna magna offers easy access to the intrathecal space and does not require surgical procedures.
To determine whether CMV vector-mediated gene transfer of pdβ-NGF can protect sensory neurons from degeneration, we used a model of pyridoxine intoxication in dogs. In high doses, pyridoxine causes a selective degeneration of large and small myelinated sensory axons in the central and peripheral nerves, resulting in numbness and loss of proprioception that manifests clinically as a sensory ataxia without weakness. The advantage of pyridoxine-induced neuropathy is the absence of systemic toxicity that often complicates analysis of treatment effect [12,13].
To analyze the effects of this experiment, observations were made by neurological examination and electrophysiological recordings. After neurological examination, loss of proprioception without other neurologic abnormalities was confirmed in only the positive control group. The neurological examination is an earlier indicator of neurotoxicity compared to other tests, so it is very useful and convenient. Among the electrophysiological recordings, M wave and H reflex were tested. The muscle may have responded as a result of a threshold stimulus (supramaximal stimulus), applied to its motor fibers. Action potentials were conducted orthodromically, resulting in the M wave. The muscle potential is the resultant activity of a true monosynaptic reflex arc and thus appropriately referred to as an H reflex. The maximal H reflex amplitudes were obtained with submaximal stimulus [18]. In a previous report, we confirmed that pyridoxine-induced neuropathy with electrophysiological recordings are related only to sensory axons in the central and peripheral nerve [10].
There are many trials of gene therapies in human neurological disorders, involving the selection of factors, vectors, delivery reagents, animal models and administration routes. In the veterinary field, especially the small animal neurological field, many neurodegenerative disorders also exist, but there are fewer studies completed. In experimental animals, such as mice and rats, there are many studies about species-specific neurotrophic factors for human medicine, but not for small animals. The results obtained in this study shall open the way for basic and applied research in veterinary neurologic areas.
Figures and Tables
Fig. 1
The nucleotide and deduced amino acid sequence of the presumed dog β-NGF (pdβ-NGF) open reading frame (ORF) along with the primers used for cloning (underlined or boxes).

Fig. 2
PC12 cells were cultured with and without the filtered supernatant and photographed at ×100 magnification. (A) Negative control group. (B) Experimental group with cells showing neurite growth.

Fig. 3
(A) Normal dorsal funiculus of L4 in the negative control group. (B) Dorsal funiculus of L4, showing disruption of axons and myelin with vacuolation in the positive control group. (C) Dorsal funiculus of L4 showed occasionally swollen axons in the gene therapy group. H&E stain, ×200.
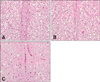
Fig. 4
(A) Normal dorsal root ganglia (DRG) of L4 in the negative control group. (B) DRG of L4 showed severe chromatolysis, vaculoation (arrowhead) and occasionally pyknotic nuclei and eosinophilic cytoplasm (arrows) in neurons in the positive control group. (C) DRG of L4 showed pyknotic nuclei and eosinophilic cytoplasm (arrows) in a few neurons in the gene therapy group. H&E stain, ×200.
Acknowledgments
This work was supported by the Brain Korea 21 program, Korean Research Foundation Grant (KRF-2006-J02902), and the Research Institute of Veterinary Science, College of Veterinary Medicine, Seoul National University.
References
1. Anderson DM, Hall LL, Ayyalapu AR, Irion VR, Nantz MH, Hecker JG. Stability of mRNA/cationic lipid lipoplexes in human and rat cerebrospinal fluid: methods and evidence for nonviral mRNA gene delivery to the central nervous system. Hum Gene Ther. 2003. 14:191–202.

2. Angeletti RH, Bradshaw RA. Nerve growth factor from mouse submaxillary gland: amino acid sequence. Proc Natl Acad Sci USA. 1971. 68:2417–2420.

3. Apfel SC. Neurotrophic factors and diabetic peripheral neuropathy. Eur Neurol. 1999. 41:Suppl 1. 27–34.

4. Callizot N, Warter JM, Poindron P. Pyridoxine-induced neuropathy in rats: a sensory neuropathy that responds to 4-methylcatechol. Neurobiol Dis. 2001. 8:626–635.

5. Cao YJ, Shibata T, Rainov NG. Liposome-mediated transfer of the bcl-2 gene results in neuroprotection after in vivo transient focal cerebral ischemia in an animal model. Gene Ther. 2002. 9:415–419.

6. Chattopadhyay M, Goss J, Lacomis D, Goins WC, Glorioso JC, Mata M, Fink DJ. Protective effect of HSV-mediated gene transfer of nerve growth factor in pyridoxine neuropathy demonstrates functional activity of trkA receptors in large sensory neurons of adult animals. Eur J Neurosci. 2003. 17:732–740.

7. Chattopadhyay M, Wolfe D, Huang S, Goss J, Glorioso JC, Mata M, Fink DJ. In vivo gene therapy for pyridoxine-induced neuropathy by herpes simplex virus-mediated gene transfer of neurotrophin-3. Ann Neurol. 2002. 51:19–27.

8. Chattopadhyay M, Wolfe D, Mata M, Huang S, Glorioso JC, Fink DJ. Long-term neuroprotection achieved with latency-associated promoter-driven herpes simplex virus gene transfer to the peripheral nervous system. Mol Ther. 2005. 12:307–313.

9. Chaudhry V, Rowinsky EK, Sartorius SE, Donehower RC, Cornblath DR. Peripheral neuropathy from taxol and cisplatin combination chemotherapy: clinical and electrophysiological studies. Ann Neurol. 1994. 35:304–311.

10. Chung JY, Choi JH, Hwang CY, Youn HY. Pyridoxine induced neuropathy by subcutaneous administration in dogs. J Vet Sci. 2008. 9:127–131.

11. Connor B, Dragunow M. The role of neuronal growth factors in neurodegenerative disorders of the human brain. Brain Res Rev. 1998. 27:1–39.

12. Hoover DM, Carlton WW. The subacute neurotoxicity of excess pyridoxine HCl and clioquinol (5-chloro-7-iodo-8-hydroxyquinoline) in beagle dogs. I. Clinical disease. Vet Pathol. 1981. 18:745–756.

13. Hoover DM, Carlton WW. The subacute neurotoxicity of excess pyridoxine HCl and clioquinol (5-chloro-7-iodo-8-hydroxyquinoline) in beagle dogs. II. Pathology. Vet Pathol. 1981. 18:757–768.

14. Hopkins AP, Gilliatt RW. Motor and sensory nerve conduction velocity in the baboon: normal values and changes during acrylamide neuropathy. J Neurol Neurosurg Psychiatry. 1971. 34:415–426.

15. Iwane M, Kitamura Y, Kaisho Y, Yoshimura K, Shintani A, Sasada R, Nakagawa S, Kawahara K, Nakahama K, Kakinuma A. Production, purification and characterization of biologically active recombinant human nerve growth factor. Biochem Biophys Res Commun. 1990. 171:116–122.

17. Mata M, Chattopadhyay M, Fink DJ. Gene therapy for the treatment of sensory neuropathy. Expert Opin Biol Ther. 2006. 6:499–507.

18. Sims MH, Selcer RR. Occurrence and evaluation of a reflex-evoked muscle potential (H reflex) in the normal dog. Am J Vet Res. 1981. 42:975–983.
19. Ullrich A, Gray A, Berman C, Dull TJ. Human beta-nerve growth factor gene sequence highly homologous to that of mouse. Nature. 1983. 303:821–825.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download