Introduction
Francisella (F.) tularensis is the causative pathogenic bacterium of tularemia. In 1911, gram negative bacilli were isolated from rodents in the Tulare area, California, and these bacilli were termed Bacterium tularense; however, these bacilli were rechristened in 1914 as Francisella tularensis [11]. F. tularensis commonly infects humans and animals, and it is primarily detected in the northern hemisphere, yet its development has been recently reported in a wider area [1,2,12,15]. The Francisella genus is classified into F. tularensis, and F. philomiragia. F. tularensis is subsequently divided into 4 subspecies; F. tularensis subsp. tularensis (type A), F. tularensis subsp. holarctica (previously subsp. palaearctica, type B), F. tularensis subsp. mediaasiatica and F. tularensis subsp. novicida [14]. The type A strain induces strong pathogenicity both in humans and animals after being transmitted by mites and rabbits, while the type B strain acts as a weak pathogen in humans and its primary transmission vehicles are mosquitoes and rodents. Type A primarily appears in North America and type B is spread in Asia and Europe [3].
The clinical features of tularemia appear as ulceroglandular/glandular, oculoglandular, pulmonary, typhoidal, gastrointenstinal tularemia, depending on the mode of transmission. Among them, the ulceroglandular/glandular type of tularemia is most frequently developed [13,18]. When human beings are infected by F. tularensis through animals, symptoms that are similar to cold are developed in 3-6 days; these symptoms are chillness, headache, fever and general pain. F. tularensis invades the body and migrates to the lymph nodes, and so patients and animals infected with this malady display inflammatory lymphadenopathy. After this the bacteria migrates to the spleen, liver, lung, kidney, colon, neural tissues and skeletal tissues. Nevertheless, the mortality of ulceroglandular/glandular tularemia is lower than 3%.
We conducted this study to characterize the immune response with using the Pohang isolate. Pohang was first isolated as a type of F. tularensis from an infected patient who had eaten a dead rabbit in Korea in 1997 [10]. In this study, we examine the types of cytokines that are expressed with time after infection in vivo, and we compared the results of infection with Pohang to that of the live vaccine strain (LVS).
Materials and Methods
Bacteria strains
F. tularensis subs. holarctica (palaearctica, Type B, LVS ATCC 29684) and a Pohang isolate (from a patient in 1997, Type B) were cultured on chocolate agar media, which including L-cysteine, at 37℃ with 5% CO2. After mass culture, the bacteria were suspended in PBS for the infection procedures.
Animal experiments
Female Balb/c mice were used at an age of 7 weeks. The animals were housed in sterile microisolator cages at Biosafety level 3 (a BSL3 animal laboratory). The mice were challenged with F. tularensis LVS and Pohang, respectively, at a dose of 2 × 104 cfu via the intradermal route. Two mice were investigated by autopsy at each time point; 0, 3, 6, 24, 48, 72, 96, 120 and 168 h after infection. The isolated organs (liver, lung, spleen, kidney and lymph nodes) were stored at -70℃ until the RNA extraction, and the sera that were separated from blood were stored at 4℃.
RNA extraction and RT-PCR
The RNA from the organs obtained at each time point was extracted with using Easy-blue solution (Intron, Korea). Each organ (30 mg) was treated with 1ml Easy-blue solution, and then it was grounded with a mortar and pestle. After treatment with 200 µl chloroform, the suspension was centrifuged at 4℃, 13,000 rpm, for 10 min. The supernatant was collected and added to a new tube, and then the same amount of isopropyl alcohol was added and this was allowed to react at room temperature for 10 min. The sample was centrifuged at 4℃, 10,000 rpm, for 10 min. The pellets were dried by adding 70% ethanol after washing. RNA-free water (50 µl/tube) was added and this was treated at 56℃ for 10 min. The extracted RNA was stored at -70℃ until use. The cDNA was synthesized using 1 µg RNA and a cDNA synthesis kit (Intron, Korea).
Cytokine PCR
Cytokine PCR was performed using the cDNA and primers that were specific to TNF-α, IFN-γ, IL-1, IL-2, IL-4, IL-10 and IL-12 (Table 1). After mixing 1 µl of cDNA, 1 µl of each primer set (10 pmol/µl), 2.5 U of i-max II Taq polymerase (Intron), 2 µl of dNTP mix (2.5 mM each) and 2 µl of 10× reaction buffer, the final volume was adjusted to 20 µl with distilled water. As for the PCR, after reacting at 94℃ for 5 min, 35 cycles of reaction were done at 94℃ for 30 sec, 60℃ for 45 sec and 72℃ for 1 min, and the final reaction was done at 72℃ for 7 min. The PCR products were assessed on 2% agarose gel and the concentrations of the bands on the photographs of the gels were measured with using a spot density meter program (FluorChem IS-8800; Alpha Innotech, USA). By applying the values, the expression ratio, based on the band intensity, was compared and analyzed.
Cytokine ELISA
The sera separated from the blood were diluted to 1/10 with PBS and these diluted sera were used for experiments. ELISA (Amersham Biosciences, USA) specific to TNF-α, IFN-γ, IL-2, IL-4, IL-10 and IL-12 was performed with following the manufacturer's protocol. The standard and diluted samples (50 µl/well) were added to the prepared plates, and these were reacted at room temperature for 2 h. After the reaction, the plates were washed 3 times with washing buffer (1 × PBS with 0.05% Tween 20). Detection antibodies conjugated with horse radish peroxidase (100 µl/well) was added again and allowed to react at room temperature for 1 h. After washing the plates 3 times, TMB (100 µl/well) solution was added, and this was allowed to react in a dark room for 30 min. After the termination of reaction by adding 100 µl of stop buffer (1 M H2SO4), the O.D values were measured at 450 nm with using a microplate reader (Model 550; Bio-Rad, USA).
Results
Mice infected with F. tularensis LVS and Pohang
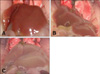
During acute infection, we found that the mice infected with F. tularensis LVS and Pohang moved slower, and so their observed activities were decreased. In regard to the changes in the organs with time, the color of the liver gradually became pale after 96 h, as compared with the uninfected control mice. The livers of the Pohang-infected mice were paler than the livers of the LVS-infected mice after 102 h (Fig. 1).
Analysis of the cytokine production and the gene expression patterns by Murine tularemia
PCR analysis of the cytokine expression: The cytokine expression was found to be increased with time in the mice challenged with F. tularensis LVS and Pohang, and the expression pattern of the cytokines in the liver was the same as that previously reported as a primary organ for F. tularensis infection (Fig. 2).
In most organs from the LVS-infected mice, the RNA expressions of the cytokines, including TNF-α and IFN-γ were gradually increased, and these expressions were elevated by approximately 2 times 168 h after infection as compared with that at the initial period. The increase of IL-10 and IL-12 with time was also detected. IL-10 began to be highly expressed in lung around 6 h after infection, and the IFN-γ expression was increased in the spleen after 24 h and this was maintained for up to 48 h. In the liver, the expressions of the TNF-α and IFN-γ RNA were detected at the highest levels after 168 h, and the expressions of the IL-10 and IL-12 RNA were increased more than 2 times in the kidney from 72 h, as compared with the initial expressions. The expression of TNF-α in the lymph nodes was elevated by approximately 2 times after 168 h, as compared with the initial period. An IL-1 expression was detected in all the organs of the normal mice, yet the difference of its expression induced by infection could not be assessed. IL-2 and IL-4 were not expressed in all the organs, except the pancreas.
For the mice infected with Pohang, the expression of IL-1 was increased more than approximately 5 times in the lung from 24 h, and the expression of TNF-α and IFN-γ was increased after 120 h. Simultaneously, the expression of IL-10 was increased about 20 times as compared with the initial period. As distinguished from LVS, it was found that a high level of a IL-1 expression, according to Pohang infection, was continuously maintained for up to 168 h. It was found that the expression of TNF-α, IFN-γ, IL-10 and IL-12 were at the highest levels in the spleen at 120 h. The expression of TNF-α and IFN-γ was found to be rapidly increased in liver after 24 h, and the highest level was reached at 120 h. The TNF-α expression was increased by approximately 50 times, and the IFN-γ expression was increased by approximately 40 times. In addition, it was found that IL-12 was also increased by approximately 16 times in comparison with the initial period. It was found that the IL-10 expression was continuously increased up to 168 h, and finally the increased level was approximately 8 times the level at the initial period. In kidney, a rapid increase of TNF-α, IFN-γ, and IL-10 was detected 120 h after infection; nonetheless it was observed that the expression of IL-12 was hardly changed. In lymph nodes, the expression of IL-12 was increased by approximately 30 times at 72 h, and simultaneously the expression of TNF-α, IFN-γ and IL-10 was slowly increased up to 168 h. The expression of TNF-α was increased by approximately 10 times, the expression of IFN-γ was increased by approximately 20 times and the expression of IL-10 was increased by approximately 60 times (Fig. 3).
Based on the results, the RNA expression levels of cytokines stimulated by the Pohang isolate were relatively higher than the levels stimulated by the LVS strain. The results also showed that liver from the mice challenged with the Pohang strain had an TNF-α expression that was increased by approximately 10 times at 24th h after infection. The levels of TNF-α and IFN-γ were rapidly elevated by approximately 40-60 times after 120 h. On the other hand, it was found that the levels of TNF-α and IFN-γ were increased by approximately 2 times in the LVS infected mice at 168 h. In addition, the expression of most cytokine was rapidly increased in the mice infected with the Pohang strain around 24th h after the initial infection, while the mice infected with the LVS strain did not show the rapid increase of cytokines, except for IL-10.
Analysis of the expression of cytokine at the protein level: The amount of cytokine produced in the blood after F. tularensis infection was measured by ELISA (Fig. 4). The production of IFN-γ protein in the mice infected with LVS was elevated slowly; 400 pg/ml IFN-γ was secreted at 72 h, and its production was subsequently decreased gradually. In the cases of the Pohang strain, IFN-γ production was continuously increased and it reached the level of 4,566 pg/ml at 120 h. Comparing this IFN-γ protein production with the levels of the RNA expression, the trends for IFN-γ for both groups of mice were similar with the highest levels being seen at around 120 h after infection. As compared with the LVS infected mice, the production of IFN-γ protein by the Pohang isolates was approximately 10 times higher. As compared with the initial period, the expression of IFN-γ by the Pohang isolates was about 40 times higher than by the LVS. It was found that the IL-10 production in the Pohang-infected mice was also substantially higher than that of the LVS-infected mice. On the other hand, the expression of IL-10 protein was hardly detected in the LVS-infected mice throughout the whole investigation, although the level of IL-10 protein was as high as 420 pg/ml in the Pohang-infected mice at 96 h. It was also found that IL-10 production was increased by about 4 times at the initial period. Nonetheless, the changes of the production of the TNF-α, IL-2 and IL-4 proteins were hardly detected with time.
Discussion
It has been reported that the mortality in animals infected with F. tularensis is influenced by various factors; the type of mouse, the infection route and the dose of the infecting bacteria. The mouse strains most suitable for F. tularensis study are C3H/HeN and Balb/c, and subcutaneous injection was reported as the most suitable route for immune reaction experiments [5]. For the F. tularensis infection among different organs, the number of bacteria is actively increased up to 5 days in the skin, lymph nodes, spleen and liver. In the cases of skin tissues, the expression of TNF-α, IFN-γ and IL-12 was previously detected 24 h after infection [17]. In our in vivo experiment on infection with LVS and Pohang, the patterns of the cytokine expression were compared and analyzed in an effort to provide the basic information of the immune responses induced by the Korean Pohang isolate. Infection with Pohang was shown to be stronger than that for LVS in Balb/c mice. With time, the fading of the liver's color in the Pohang-infected mice was more distinctive, and the movement of the mice became slower. In the preliminary experiments on mice infected with 2 × 105 cfu of bacteria, it was confirmed that all the Pohang-infected mice died within 96 h, and the LVS-infected remained alive for up to 196 h (data not shown).
It has been reported that the important immune response for F. tularensis infection is cell-mediated immunity, and TNF-α and IFN-γ are expressed during the initial period of infection [4,8]. Generally, skin lesions as early immune responses are shown 3-5 days after infection of F. tularensis. TNF-α, IFN-γ and IL-12 are expressed to control the infection during the innate immune response [19]. In this experiment, we confirmed that TNF-α and IFN-γ were markedly increased from 120 h in the F. tularensis LVS-infected mice. However, in the F. tularensis Pohang-infected mice, the expressions of TNF-α and IFN-γ were elevated rapidly from 24 h, and they were increased by approximately 40 times at 120 h after infection as compared with the initial period. IL-12 was also found to be increased by approximately 20 times in the F. tularensis Pohang-infected mice. This may suggest that the immune reaction progressed more rapidly in mice infected with Pohang as compared with the mice infected with LVS. Similar results were also obtained in other organs.
In our experiments on infected animals, it was confirmed that IL-1 was expressed from the initial infection period in all the organs. Such a fact shows its importance in association with the inflammatory reaction stimulated by other bacteria, and the results from the previously reported experiments that used Listeria monocytogenes showed the survival rate of the group treated with anti-IL-1 antibody was different from that of the untreated group; it could be considered that IL-1 may also play an important role in the initial period of infection [16]. In addition, it has been reported that the increase of IL-1β and IL-8 in F. tularensis-infected human monocytes started 2 h after infection [6]. Regarding IL-2 and IL-4, they were not expressed in any organ except the spleen, and this result was in agreement with the results reported by others [7].
IL-10, the impeding inflammatory reaction and cell-mediated immunity have been reported to be down-regulators that suppress the release of TNF-α and IL-12 from macrophages. In our experiment, the pattern was that the expression of IL-10 was also increased by a small degree with time, and this increase after F. tularensis infection might play a different role than regulating pro-inflammatory cytokine [9].
Based on the above results, it can be suggested that F. tularensis induces a Th1-mediated immune reaction by the expression of cytokines, including TNF-α, IFN-γ, IL-1 and IL-12, and the immune responses caused by the Pohang isolate from a Korean patient progressed rapidly in comparison with that caused by LVS. Our study about the pathogenicity and the immune response during infection of F. tularensis should help to further characterize all the isolates in Korea, including the Pohang isolate.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download