Introduction
Nitric oxide (NO) is an important intracelluar and intercellular signaling molecule that is involved in regulation of physiological and pathological mechanisms in cardiovascular, nervous and immune systems. NO plays many roles in living organisms, including regulation of muscle tone in vascular systems and acting as a biological mediator that functions in a fashion similar to neurotransmitters in the nervous system. In addition, NO is an important host defense effector molecule in the immune system [2]. Conversely, NO can also act as a cytotoxic agent in pathological processes [4].
At physiological concentrations, NO inhibits proinflammatory platelet aggregation, integrin-mediated adhesion, and proinflammation induced gene expression, which are all factors that control vascular inflammation and oxidative injury. However, at high concentrations, NO and NO2- can exert pathogenic properties due to the production of a more toxic metabolite, peroxinitrite (ONOO-), which causes a reversal of the positive effects of NO [7].
NO is generated by the conversion of L-arginine to L-citruline in the presence of the family of nitric oxide synthases (NOSs). Three isoforms of NOS have been found in various cell types. Both endothelial NOS and neuronal NOS are constitutive isoforms that play housekeeping roles by producing physiological concentrations of NO. Conversely, inducible NOS (iNOS) has the potential to synthesize high concentrations of NO during inflammatory processes in various types of cells such as endothelial cells, hepatocytes, monocytes, mast cells, macrophages and smooth muscle cells that have been stimulated by cytokines or bacterial products [28].
Expression of the iNOS gene in macrophages is under the control of several transcription factors, including nuclear factor (NF)-κB [29]. NF-κB is functional as a hetero- or homo-dimeric form of proteins in the Rel family, such as RelA (p65), RelB, cRel, p50 and p52 and is sequestered in the cytoplasm by binding to IκB proteins such as IκBα, IκBβ, IκBε, p105 and p100. Lipopolysaccharide (LPS) is a major component of the outer membranes of Gram-negative bacteria that can trigger a variety of inflammatory reactions by binding to its specific receptor, Toll-like receptor 4 [1]. Signalling components downstream of the receptor, in turn, activate the IκB kinase (IKK) complex [12]. Activation of the IKK complex results in phosphorylation of IκB, which masks its signal and results in ubiquitination, ultimately leading to proteasome-mediated degradation [19,25]. IκB degradation then unmasks the nuclear localization signal motif of NF-κB, which allows the transcription factor to move into the nucleus where it binds to the promoter region of immune and inflammatory genes such as iNOS, thereby regulating transcription [8,26].
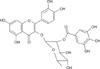
Quercetin 3-O-β-(2"-galloyl)-rhamnopyranoside (QGR) is a naturally occurring quercitrin gallate (Fig. 1), which is a polyphenolic compound that was originally isolated from Persicaria lapathifolia. It has been reported that QGR inhibits the iNOS expression induced by LPS treatment in macrophage RAW 264.7 cells by inhibiting nuclear translocation of NF-κB [14]. However, it is not known if QGR inhibits iNOS expression and NO production in vivo. Therefore, we conducted this study to determine if QGR exerts an inhibitory effect on iNOS expression and NO production induced by LPS treatment in Balb/c mice.
Materials and Methods
Reagents
LPS (E. coli O55:B5) was purchased from Sigma-Aldrich (USA). The sequences of primer pairs for iNOS and GAPDH were synthesized by Bioneer (Korea). The other commercially purchased reagents were as follows: RNAiso reagent and a primeScript 1st strand cDNA synthesis kit from TaKaRa (Japan), Pro-prep and Pro-measure from iNtRON Biotechnology (Korea), anti-iNOS IgG from Santa Cruz Biotechnology (USA), anti-β-actin IgG and anti-rabbit IgG from Cell Signaling Technology (USA), polyvinylidene difluoride membrane from Millipore (USA). QGR (purity, > 98%) was isolated from P. lapathifolia [14].
Animal experiment
Seven week-old male Balb/c mice were purchased from Daehan Biolink (Korea). All animals were maintained under constant environmental conditions (temperature: 21-24℃, relative humidity: 35-65%, 12-h light/12-h dark cycle). All animal experiments were performed in accordance with an interim guideline approved by the Institutional Animal Care and Use Committee of the Laboratory Animal Research Center in Chungbuk National University.
A total of 15 mice were randomly divided into 3 groups. Mice in group 1 were treated with vehicle as a control, mice in group 2 were treated with LPS (10 mg/kg) intraperitonially as a treatment control and mice in group 3 were treated with QGR and LPS. QGR was administered to the mice once a day for 3 days at a dose of 10 mg/kg by gavage prior to LPS treatment. Six hours after LPS injection the mice were sacrificed under ether anesthesia.
Measurement of NO concentration
After being sacrificed, the blood was collected from the abdominal vein and then centrifuged at 3,000 rpm for 20 min to obtain the serum. To measure the NO in the serum, 100 µl of serum was mixed with the same volume of Griess reagent (1% sulfanilamide in 5% phosphoric acid and 0.1% naphthylethylenediamine dihydrochloride in water) and then incubated for 10 min at room temperature. The optical densities were then measured at 540 nm using an ELISA reader (V-MAX 220 VAC; Molecular Devices, USA).
Reverse transcription-polymerase chain reaction
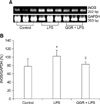
Total RNA was extracted from the livers of the mice using an RNAiso reagent kit according to the manufacturer's guides. Five µg of the total RNA were then used for reverse transcription to generate cDNA using a cDNA synthesis kit. The cDNA was then used as a template for PCR reactions with primers specific for iNOS or GAPDH. The sequences of the primers used to amplify iNOS were 5'-CCTCCTCC ACCCTACCAAGT-3' and 5'-CACCCAAAGTGCTTCA GTCA-3' (Gene Bank Accession No. NM010927), and the sequences of the primers used to amplify GADPH were 5'-AACGGATTTGGTCGTATTGG-3' and 5'-AGCCTTC TCCATGGTGGTGAAGAC-3' (Gene Bank Accession No. NM017008). Each cDNA was amplified by subjecting the reaction mixture to the following conditions: 35 cycles of denaturation at 95℃ for 30 sec, annealing at 60℃ for 30 sec, and extension at 72℃ for 1 min. The amplified cDNA was then separated on 1.5% agarose gels and visualized by staining with ethidium bromide. The relative intensities of the iNOS bands were then normalized to the corresponding GAPDH band intensities. The results were then analyzed using the Quantity One program (Gel Doc EQ; Bio-Rad, USA).
Western blot analysis
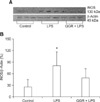
Total protein was extracted from the livers of mice using a Pro-prep kit according to the manufacturer's guides (iNtRON Biotechnology, Korea). One-hundred µg of protein were then denatured by boiling at 95℃ for 5 min in sample buffer (0.5 M Tris-HCl, pH 6.8, 10% sodium dodecyl sulfate (SDS), 0.36% glycerol, 0.06% 2-ME and 12% bromophenol blue). The samples were then separated by electrophoresis on 7.5% SDS-polyvinylamide minigels, after which they were transferred to polyvinylidene difluoride membranes in solution (25 mM Tris, 192 mM glycine in 20% methanol, pH 8.3). Next, the samples were blocked for 1 hr with 5% skim milk in Tris-buffered saline Tween 20 (TBST, 25 mM Tris, 150 mM NaCl, 0.05% Tween 20), after which the membranes were incubated overnight with 1:250 dilutions of rabbit anti-murine iNOS polyclonal antibody or 1:1,000 dilutions of rabbit anti-β-actin polyclonal antibody at 4℃. The membranes were then washed with TBST, after which they were incubated for 1 h with 1:1,000 dilutions of goat anti-rabbit IgG conjugated with horseradish peroxidase at room temperature. The relative intensities of the iNOS bands were then normalized to the corresponding β-actin band intensities. The films were then scanned and analyzed using the Quantity One program (Gel Doc EQ; Bio-Rad, USA).
Histopathology
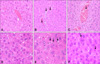
Liver tissues were fixed with 10% phosphate buffered formalin and then processed following routine histological techniques. After paraffin embedment, 4 µm sections were stained with hematoxylin and eosin and then subjected to histopathologic evaluation. The histological changes were quantitatively analyzed using an index of the severity of tissue injury. The index was based on neutrophil infiltration, which was determined by counting the polymorphonuclear neutrophils (PMN) in 10 randomly selected high-power fields (×400). The index was expressed as the mean ± SD.
Results
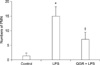
Effect of QGR on NO production in serum
As shown in Fig. 2, the concentration of NO significantly increased from 10 µM to 60 µM in response to treatment with LPS. However, pretreatment with QGR inhibited the increase in NO that was induced by LPS by approximately 50%.
Effect of QGR on iNOS mRNA expression in the liver
As shown in Fig. 3, the expression of iNOS mRNA was approximately 77% of that of the expression of GAPDH in control cells. However, the expression of iNOS mRNA increased to 103% of that of the expression of GAPDH in control cells in response to treatment with LPS. When mice were pretreated with QGR, the expression of iNOS mRNA in the LPS group was 83% of that of the expression of GAPDH in the control group.
Effect of QGR on iNOS protein expression in the liver
As shown in Fig. 4, the level of iNOS protein expression in control cells was approximately 25% of that of the expression of β-actin in the controls. In addition, iNOS protein expression in LPS treated mice increased to 80% of that of the expression of β-actin in the controls. However, pretreatment with QGR inhibited the increased expression of iNOS protein that was induced by LPS to 50% of the expression of β-actin.
Effect of QGR on PMN infiltration in the liver
To evaluate the histological changes in response to treatment, tissue slides were made from liver samples. Almost no infiltration of PMN was observed in the livers of mice that were subjected to the control treatment. However, there was obvious infiltration of PMN in the portal veins and sinusoids of livers from mice that were treated with LPS (Fig. 5). In addition, many necrotic cells were observed in some areas of the livers of LPS treated mice. Pretreatment with QGR significantly decreased the number of infiltrated PMN and necrotic cells in the livers of mice that were treated with LPS (Fig. 6).
Discussion
NO has both protective and destructive effects on biological features. It acts as a neurotransmitter and is an important host defense effector, as well as a regulator of blood pressure [2]. Conversely, it has a free radical structure and acts as a cytotoxic agent in pathological processes [4].
Many types of cells express iNOS as part of the host defense against bacterial, parasitic and viral pathogens [5]. This expression leads to the formation of NO radicals and its reaction products, S-nitrosothiols or ONOO-, in the host cells or the invading microbe itself. iNOS expression in macrophages is activated by particular inducers, after which it participates in the pathology of inflammatory diseases such as atherosclerosis, rheumatoid arthritis, diabetes, septic shock, and cell death [6,10]. Accordingly, several reports have shown that iNOS inhibitors ease the symptoms of arthritis, ulcerative colitis and autoimmune diseases [24].
Inhibition of NF-κB activation is considered to be important when designing iNOS inhibitors because NF-κB activation is the primary regulatory step involved in iNOS expression [3,5]. Recently, a large number of substances derived from plants have been evaluated to determine if they could inhibit the NF-κB pathway. These substances include lignans such as manassantins and saucernetin [22], sesquiterpenes such as celastrol [16], costunolide and celaohanol [13], diterpenes such as excisanin and kamebakaurin [11], triterpenes such as avicin [19] and oleandrin [20], and polyphenols such as resveratrol [18], epigallocatechin gallate [17] and quercetin [27].
QGR is a naturally occurring quercitrin gallate, which is a polyphenolic compound that was originally isolated from Persicaria lapathifolia (Polygonacease) [14]. It has been reported that QGR inhibits NADPH oxidase complex-mediated superoxide production in unopsonized zymosan-stimulated human monocytes through its weak ability to scavenge oxygen/nitrogen radical species such as superoxide and NO [15]. In addition, QGR has been reported to inhibit iNOS expression induced by LPS treatment in macrophage RAW 264.7 cells by inhibiting nuclear translocation of NF-κB [14].
Quercetin is an aglycone of QGR that has been reported to inhibit LPS-dependent production of iNOS mRNA and to decrease the release of NO in macrophage RAW 264.7 cells [21]. In addition, quercetin has been shown to exert anti-inflammatory effects by acting on IKK complex as a mixed type of inhibitor, which suggests that its bindings site overlaps both the ATP and IκBα binding pockets on the enzyme [23]. However, since QGR does not inhibit LPS-mediated IκBα phosphorylation, the effects of QGR on LPS-mediated NF-κB activation must function through a different inhibitory mechanism from its aglycone, quercetin [14].
In the present study, we demonstrated that QGR suppressed iNOS mRNA and protein expression in the liver and reduced the serum NO concentration of mice that were challenged by LPS. In addition, we found that QGR attenuated the infiltration of PMN and hepatocytic necrosis. Taken together, these results indicate that QGR exerts its antiinflammatory activity by inhibiting the iNOS-NO pathway, and that it has therapeutic potential for the treatment of a wide range of inflammatory diseases.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download