Feline herpesvirus type 1 (FHV-1) is the most frequent cause of conjunctivitis, and it also induces corneal ulcers, stromal keratitis, corneal sequestration and keratoconjunctivitis sicca in cats [14]. Chlamydophila (C.) felis (previously called Chlamydia psittaci) is another major conjunctival pathogen [13]. Feline calicivirus (FCV) is an unlikely and minor primary conjunctival pathogen [9]. It is considered to be the most common upper respiratory tract disease (URTD) associated pathogen in cats [16].
The prevalence of FHV-1, FCV and C. felis in cats throughout the world has been frequently reported [1-3,5,6,12,15,16]. However, there have been relatively fewer of these studies for clinically normal cats in a shelter environment [1,5,11] and no such research has been done in Korea. The purpose of this study is to identify the prevalence of FHV-1, FCV and C. felis in clinically normal cats of a Korean animal shelter by performing multiplex reverse transcription-polymerase chain reaction (RT-PCR)/PCR.
Samples were collected from one animal shelter (Yangju, Korea) during January 2005. The animals were held at least 30 days and euthanasia was performed because of illness or injury, or insufficient available space. The shelter was traditional with no quarantine for incoming cats, but the cats having diseases were separated from the main shelter.
A total of 78 cats were examined before they underwent euthanasia. Based on their history and ophthalmic (using slit lamp biomicroscopy) and physical examinations, they had clinically normal eyes and they had no specific clinical signs of URTD. All the cats were domestic short-haired or long-haired. The number of male cats examined was 40 and 38 were females. The cats' age and neuter and vaccination status could not be exactly determined. For each cat, specimens were obtained using three sterile cotton tipped swabs in the conjunctival sac of both eyes and the oropharynx, respectively. These were preserved in 2 ml phosphate buffered saline (PBS, 0.01 M NaPO4, pH 7.0) and they were immediately sent to the laboratory within 2 h and then stored at -70℃ until they were assayed. Before the subsequent nucleic acid extraction, the specimens that were separately obtained from the three sites were thoroughly mixed.
One commercial vaccine (Felocell CVR-C; Pfizer Animal Health, USA) was used as a positive control. It was the only licensed vaccine for preventing FHV-1, FCV and C. felis in Korea at the time of the study. Sterile distilled water without nucleic acids was used as a negative control. Both the controls were subjected to nucleic acid extraction and PCR. The nucleic acids were extracted from specimens by using the Viral Gene-spin kit (Intron Biotechnology, Korea) according to the manufacturer's instructions.
Three pairs of oligonucleotide primers were used for the amplifying reaction. We used the previous designed primer sequences by Sykes et al. [16]. HerpF (5'-GACGTGGTGAATTATCAGC-3') and HerpR (5'-CAACTAGATTTCCACCAGGA-3') amplify a 292-base pair (bp) region in the thymidine kinase (TK) gene of FHV-1. ChlaF (5'-ATGAAAAAACTCTTGAAATCGG-3') and ChlaR (5'-CAAGATTTTCTAGACTTCATTTTGTT-3') amplify a 1069-bp fragment in the outer membrane protein gene of C. felis. CalcapF (5'-TTCGGCCTTTTGTGTTCC-3') and CalcapR (5'-TTGAGAATTGAACACATCAATAGATC-3') amplify a 673-bp conserved region in the capsid protein gene of FCV.
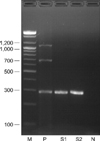
Multiplex RT-PCR/PCR was performed according to a previous study [16]. Each 15 µl of reaction products was electrophoresed through a 1.5% agarose gel and the proteins were stained with ethidium bromide; the appropriate molecular weight markers (100-bp DNA ladder; Bioneer, Korea) are adjacent to them. The positive control included the extracted nucleic acid of the commercial strains in the vaccine and the negative control consisted of all the RT-PCR/PCR reagents except the nucleic acid; these were included in each reaction.
When 78 clinically normal cats were examined, 49 (63%) were positive for FHV-1. Of the 40 male cats examined, FHV-1 was detected in 23 cats (58%) and 26 of the 38 female cats (68%) were positive to FHV-1. However, C. felis and FCV were negative in all the specimens we obtained. The products resulting from amplification of FHV-1, C. felis and FCV in two specimens are shown in Fig. 1.
Many studies have reported on the prevalence of FHV-1, C. felis and FCV in clinically normal or abnormal cats [1-3,5,6,8,12,15,16]. There were various detection rates of FHV-1 according to the breeding sites, the countries and the cats' clinical status. The prevalence of FHV-1 was ranged from 0 to 52% for clinically normal cats of a breeding cattery or shelter in Sweden [6], the USA [2,11,15] and European countries [5]. The prevalence of FHV-1 for client-owned normal cats was 5.9% [10] and this was 12% [15] in the USA. The percentage of cats with diseases at a breeding cattery or shelter in European countries [5] and the USA [1] ranged from 0 to 41%. For client-owned abnormal cats, FHV-1 was detected in 4.5% to 76.3% of the cats in Japan [3,9], the USA [2,3,10,15], Australia [16] and Italy [12]. Based on these findings, the FHV-1 positive rates of the shelter cats were generally higher than those of the client owned-cats or the breeding cattery cats. Especially, many shelter cats without diseases had a higher prevalence than did the clinically abnormal client-owned cats. We think that the results are due to that many shelter cats have been infected with FHV-1 and they remain subclinical carriers after recovery. At least 80% of infected cats remain latently infected and 29% of them shed virus spontaneously [4]. Thus, FHV-1 has been frequently detected in latently infected cats that are without clinical signs. Also, those cats that live in a colony like an animal shelter, an animal hospital and a multi-cat household can be more frequently exposed to FHV-1.
Among the shelter cats around the world, those in Korea have the highest prevalence of FHV-1 (63%). This result may be due to some reasons. The first is the difference in the frequency and level of stressful events that cats are exposed to in an animal shelter. Stress as simple as moving a cat into a new environment can convert a latent infection to an active infection in a few days [11]. Virus shedding continues for 1-2 weeks around 1 week after a stressful event [6]. Thus, in this study, cats may have the greatest risk of infection through more exposure to reactivated virus from carriers because the Korean housing environment may be more stressful than that in other countries. The second reason is there is a high shedding rate for incoming cats at the time of first entry to the shelter. However, in other study, the shedding rate was much lower than the rate after 1 week in the shelter [11]. To certify this possibility, it will be necessary to investigate the proportion of cats that already shed FHV-1 at the time they are relinquished.
It was previously reported that the prevalence of C. felis in clinically normal cats of a breeding cattery or shelter was only 3% in European countries [5]. For cats with diseases and that are in the same environment, the prevalence of C. felis ranged from 0 to 10% in the USA [1] and European countries [5], whereas that of client owned abnormal cats was 11.5%, 20% and 59.1% in Australia [16], Italy [12] and Japan [3], respectively. According to these previous reports, clinically normal cats had a much lower prevalence of C. felis than that of abnormal cats. Unlike FHV-1, those cats in a shelter or a breeding cattery had a low detection rate of C. felis. These results indicate that the clinical signs of URTD or ocular disease are correlated with infection of C. felis and they are not related to the housing environment. C. felis was not detected in our study.
Generally, normal cats without disease have a lower detection rate of FCV, ranging from 1 to 29% [5,8,9,11], than abnormal cats, ranging from 0 to 47% [1,3,5,9,16]. FCV was rarely detected in two studies that focused on clinically normal cats (1% in Japan and 2.6% in Sweden) [6,9]. One previous study even showed that FCV was not detected from clinically abnormal cats in some shelters [1]. In our present study, FCV was not detected at all. This result may be due to several reasons. First is a possibility that all the examined cats were not truly infected with FCV. The second possible reason is that the chronic infected cats were not shedding virus. Last, it has been reported that the lower detection rate of FCV is due to some problems for detecting virus on in the feline mucosal swabs, that is, the very small number of virus particles, the presence of RNase in the mucosa and the FCV's genetic variability [16]. We can not exactly determine how these factors contribute to a low detection rate.
This study demonstrated that many cats of an animal shelter in Korea, as well as other countries, remain latently infected with FHV-1. These cats would be sources of infection after returning to society. Thus, proper management by veterinarians, cleaning protocols, a low stress environment and proper cage design are necessary in an animal shelter. Especially, it may be important to make a less stressful environment through decreasing the extent of crowding. Although vaccination with FHV-1 and FCV cannot lead to prevention of infection and viral shedding, this may reduce the overall severity of disease [16]. Thus, it is necessary to routinely vaccinate the individual shelter cats and to keep incoming cats from infected sources such as shelters and breeding catteries.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download