Abstract
We investigated the expression of aquaporin 1 (AQP1) in tissues from canines with an inherited anomaly that causes their erythrocytes to have high K+. Northern blot analysis revealed abundant AQP1 expression in lung and kidney, though little expression was found in spleen. Using anti-C-terminus for dog AQP1, abundant expression was shown in kidney, trachea, and eye, but little expression was shown in pancreas and cerebrum, indicating that AQP1 expression in canine tissues is similar to that noted in other mammals.
Aquaporins (AQP) are expressed in a variety of water-transporting epithelia and in many other tissues, in which they play an important role in facilitating water transport across the cell membrane. The AQP1 water channel was first isolated from human red blood cells (RBCs) [2] and was characterized to function as a water channel with high osmotic water permeability [15]. In human erythroleukemia HEL and K562 cells, AQP1 expression has been induced by sodium butyrate, which is a strong inducer of erythroid differentiation [16]; a putative butyrate-response element has been identified in the promoter sequence of the human AQP1 gene. AQP1 expression has been induced by dimethyl sulfoxide and corticosteroids in mouse erythroleukemia MEL cells [12]. Although a great deal of information is known about AQP1 expression in humans and rodents [1,8], information is quite limited in canines. Previously, we determined the cDNA sequence in canine erythroblasts and undertook functional analysis of canine AQP1 using Xenopus oocytes [5]. Mature RBCs from carnivores usually lack a Na+-K+-ATPase, and their cation composition is high Na+ and low K+ (LK), just like plasma. However, some dogs in the Japanese Shiba dog family have been found to possess a Na+-K+ pump, and their RBC cation composition is high K+ (HK) and low Na+, like other mammals [10]. We previously reported that the K+-Cl- co-transporter plays an important role in regulatory volume decrease (RVD) in HK RBCs when they are swollen in hypo-osmotic condition; the Na+-Ca2+ exchanger plays the same role in LK RBCs [3]. In each case, water permeation mediated by AQP1 may cooperate with each transporter to achieve RVD. In this study, we investigated AQP1 expression in tissues from canines with inherited HK erythrocytes using Northern blot and Western blot analyses. We then compared ours results with those found in normal LK dogs and other animals.
All experiments met the guidelines of the Laboratory Animal Care Committee of Azabu University. For the Northern blot analysis, 10 µg of mRNA from each tissue sample, purified with oligo-(dT) cellulose, was subjected to standard electrophoresis on 1% agarose gels containing 1 × MOPS buffer with formaldehyde. The gels were transferred to a Hybond-N filter (GE Healthcare Bio-Sciences, Japan) and hybridized with a probe containing the coding sequence of the dog AQP1 from nt428-816. The DNA fragment used as a probe was amplified by RT-PCR with the primer set listed in Table 1. Radioactivity was visualized by autoradiography using the FLA-2000 digital imaging system (Fuji Film, Japan). The dog glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) fragment was used as a control for RNA integrity. Signal intensity for each sample was standardized using that of GAPDH.
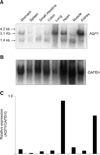
Fig. 1 shows the Northern blot of AQP1 in HK dog tissues (A). Lung, heart, and kidney demonstrated an intense signal compared with other tissues. Each sample represented the major transcripts of approximately 3.1 kb and/or 1.4 kb signals. Skeletal muscle and small intestine composed the predominant signal in the 1.4 kb band. Signal intensity of GAPDH varied among tissues, despite loading of an equal amount of mRNA across tissues (B). Therefore, relative AQP1 expression was standardized by that of GAPDH in each tissue. Standardization revealed abundant AQP1 expression in lung and kidney, but little in spleen (C).
There were some differences in the mRNA transcriptional pattern between high K dogs and rats. Unlike rat tissues, there was no 4.2 kb band in any HK dog tissue preparation. The 1.4 kb band was predominant in skeletal muscle and small intestine of HK dogs, whereas only skeletal muscle exhibited a predominant 1.4 kb band in rats [13]. In rats, AQP1 expression was clearly detected in spleen [13], though AQP1 expression in HK dog spleen was unpronounced. To investigate AQP1 protein expression in various HK dog tissues, anti-dog AQP1 serum was prepared with the peptide antigen designed according to the C-terminus amino acid sequence of dog AQP1 (RVKVWTSGQVEEYEL; residues 243-257) [5].
The membrane of each tissue was prepared for Western blot as reported by Denker et al. [2]. Protein concentration was determined by the BCA method, and the protein was used for Western blot analysis.
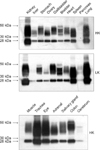
Fig. 2 shows the distribution of AQP1 in HK and LK tissues. We found that AQP1 was very abundant in kidney, lung, trachea, and eye, but was scarce in pancreas and cerebrum. This finding is, as a whole, consistent with that of reported ribonuclease protectin and Western blot assays [14,17]. The strong Western blot signal in spleen, which was weak on Northern blot, was considered to be due to abundance of RBC membrane proteins in the spleen. There was no significant difference in AQP1 expression between the HK and LK tissues examined (Fig. 2A).
In this report, we investigated the expression of AQP1 in canines possessing an inherited trait that causes their erythrocytes to have high K+. We previously reported the high incidence of HK dogs in some breeds in Korea and Japan, but no HK dogs have been found in other areas of East Asia [4]. Interestingly, these HK cells exhibit characteristics different from normal LK cells in several ways. Firstly, HK cells have activated Na+-dependent amino acid transport due to the Na+ driving force created by the Na+-K+ pump. This results in abnormal accumulation of three amino acids (Asp, Glu, and Gln) and glutathione [6,11]. The volume of HK cells is greater than that of LK cells, the lifetime of the HK cells is half that of LK cells, and some of the glycolytic enzymes exhibit an immature type of isozyme [7]. These characteristics have been shown to be inherited in an autosomal recessive manner [9]. The above abnormalities suggest that there are defects in the differentiation or maturation of HK cells. This dimorphism in RBC intracellular cation composition causes the differential regulatory volume decrease seen when the cells are swollen in a hypo-osmotic environment, despite the fact that there is no difference in AQP1 expression between HK and LK dogs. Still, the reason why the Na+-K+ pump is retained on HK RBCs is unknown. Analysis of HK dogs may shed light on the evolution of carnivore erythrocytes. Further investigation in HK dogs possessing unique RBCs will provide more insight into the physiology of water homeostasis in canines.
Figures and Tables
Fig. 1
Northern blot analysis of AQP1 expression in dog tissues (A). Each 10 µg sample of mRNA was purified, electrophoresed, and blotted. Hybridization of this blot with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to ensure RNA integrity is also shown (B). AQP1 expression of each tissue was standardized using the signal intensity of GAPDH (C).
References
1. Burg MB, Kwon ED, Kültz D. Regulation of gene expression by hypertonicity. Annu Rev Physiol. 1997. 59:437–455.

2. Denker BM, Smith BL, Kuhajda FP, Agre P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J Biol Chem. 1988. 263:15634–15642.

3. Fujise H, Higa K, Kanemaru T, Fukuda M, Adragna NC, Lauf P. GSH depletion, K-Cl cotransport, and regulatory volume decrease in high-K/high-GSH dog red blood cells. Am J Physiol Cell Physiol. 2001. 281:C2003–C2009.
4. Fujise H, Higa K, Nakayama T, Wada K, Ochiai H, Tanabe Y. Incidence of dogs possessing red blood cells with high K in Japan and East Asia. J Vet Med Sci. 1997. 59:495–497.

5. Higa K, Ochiai H, Fujise H. Molecular cloning and expression of aquaporin 1 (AQP1) in dog kidney and erythroblasts. Biochim Biophys Acta. 2000. 1463:374–382.

6. Inaba M, Maede Y. Increase of Na+ gradient-dependent L-glutamate and L-aspartate transport in high K+ dog erythrocytes associated with high activity of (Na+, K+)-ATPase. J Biol Chem. 1984. 259:312–317.

7. Inaba M, Maede Y. Inherited persistence of immature type pyruvate kinase and hexokinase isozymes in dog erythrocytes. Comp Biochem Physiol B. 1989. 92:151–156.

8. Jenq W, Cooper DR, Bittle P, Ramirez G. Aquaporin-1 expression in proximal tubule epithelial cells of human kidney is regulated by hyperosmolarity and contrast agents. Biochem Biophys Res Commun. 1999. 256:240–248.

9. Maede Y, Inaba M. Energy metabolism in canine erythrocytes associated with inherited high Na+- and K+-stimulated adenosine triphosphatase activity. Am J Vet Res. 1987. 48:114–118.
10. Maede Y, Inaba M, Taniguchi N. Increase of Na-K-ATPase activity, glutamate, and aspartate uptake in dog erythrocytes associated with hereditary high accumulation of GSH, glutamate, glutamine, and aspartate. Blood. 1983. 61:493–499.

11. Maede Y, Kasai N, Taniguchi N. Hereditary high concentration of glutathione in canine erythrocytes associated with high accumulation of glutamate, glutamine, and aspartate. Blood. 1982. 59:883–889.

12. Moon C, King LS, Agre P. Aqp1 expression in erythroleukemia cells: genetic regulation of glucocorticoid and chemical induction. Am J Physiol. 1997. 273:C1562–C1570.
13. Moon C, Preston GM, Griffin CA, Jabs EW, Agre P. The human aquaporin-CHIP gene. Structure, organization, and chromosomal localization. J Biol Chem. 1993. 268:15772–15778.

14. Nielsen S, Smith BL, Christensen EI, Agre P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci USA. 1993. 90:7275–7279.

15. Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992. 256:385–387.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download