Abstract
The present study was conducted to examine the morphology and antigenicity of Photobacterium damselae subsp. piscicida by culturing the bacterium in vivo in the peritoneal cavity of sea bass (Dicentrarchus labrax) within dialysis bags with either a low molecular weight (LMW) cut-off of 25 kDa or a high molecular weight (HMW) cut-off of 300 kDa. Differences were observed in the growth rate between the bacteria cultured in vivo or in vitro. Bacteria cultured in vivo were smaller and produced a capsular layer, which was more prominent in bacteria cultured in the HMW bag. Antigenicity was examined by Western blot analysis using sera from sea bass injected with live Ph. d. subsp. piscicida. The sera recognised bands at 45 and 20 kDa in bacteria cultured in vivo in the LMW bag. Bacteria cultured in vivo in the HMW bag did not express the 45 kDa band when whole cell extracts were examined, although the antigen was present in their extracellular products. In addition, these bacteria had a band at 18 kDa rather than 20 kDa. Differences in glycoprotein were also evident between bacteria cultured in vitro and in vivo. Bacteria cultured in vitro in LMW and HMW bags displayed a single 26 kDa band. Bacteria cultured in the LMW bag in vivo displayed bands at 26 and 27 kDa, while bacteria cultured in vivo in the HMW bag possessed only the 27 kDa band. These bands may represent sialic acid. The significance of the changes observed in the bacterium's structure and antigenicity when cultured in vivo is discussed.
Photobacterium damselae subsp. piscicida, the causative agent of pasteurellosis, has been reported in yellowtail (Seriola quinqueradiata) in Japan [20]; hybrid striped bass (striped bass Morone saxatilis × M. chrysops) in the southern United States [16]; and in sea bass (Dicentrarchus labrax), sea bream (Spraus aurata), hybrid sea bass (Morone saxatilis × M. chrysops) and sole (Solea senegalensis) in the Mediterranean region [26,29,30]. The disease has exacted considerable economic losses to the marine aquaculture industry in these regions, which has necessitated the development of an efficient vaccine [6,21,22,25,27]. In seeking to better understand the natural antigenic characteristics of the pathogen, the bacterium has been grown under "near in vivo" conditions. These conditions include iron limitation, which leads to production of siderophores [5,10-12,17,23]; glucose, which stimulates capsule formation [5,7]; or in various concentrations of NaCl, which appears to affect the antigenicity of the bacterium [28].
The effect of different culture conditions on the antigenicity of extracellular products (ECP) of the bacterium, which are responsible for most of the lethal effects observed in fish, have also been examined [1,3].
Identification and characterization of the antigens expressed by the bacterium during infection is important, not only for understanding the bacterium's pathogenesis, but also for developing effective vaccines and diagnostic tests [8,14]. Garduño et al. [14] examined the growth and expression of antigens by Aeromonas salmonicida after culturing the bacterium in specialized chambers implanted within the peritoneal cavity of rainbow trout, Oncorhynchus mykiss. The study represented the first fish pathogen to be cultured in vivo. More recently, the expression of antigens by Ph. d. subsp. piscicida was examined following bacterial growth in dialysis tubing implanted in the peritoneal cavity of sea bass [4]. These authors identified a number of novel antigens associated with the bacterium and ECP, and demonstrated that antigens induced under iron-restriction were conserved on bacteria grown in vivo.
The aim of the present study was to examine the morphology and antigenicity of P. damselae subsp. piscicida grown in vivo. The bacteria were cultured in dialysis tubing in the peritoneal cavity of sea bass. Two different molecular weight cut-offs (MWCO) of 25 kDa and 300 kDa were used. Morphological variations were ascertained using transmission electron microscopy (TEM), the presence of novel proteins and antigens was detected using sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis using sera from infected sea bass, and carbohydrate profiles were determined. These results were compared with the bacteria grown in vitro.
Photobacterium damselae subsp. piscicida isolate I752, which had been obtained from diseased sea bream in 1996, was identified using biochemical, morphological and serological analyses. The presently used bacterial stain was selected after investigating the in vitro culture-dependent variation in the molecular weights of expressed antigens [19].
Bacteria were routinely cultured in tryptone soya broth (TSB) or tryptone soya agar (TSA) at 22℃ for 16 h. They were harvested and washed three times with sterile phosphate buffered saline (PBS: 0.02 M NaH2PO4.2H2O, 0.02 M Na2HPO4.2H2O, 0.15 M NaCl, pH 7.2) at 2,900 × g for 20 min at 4℃ and resuspended in PBS. The concentration of the bacteria was determined spectrophotometrically at 610 nm and adjusted to an absorbance of 1.0. The number of live bacteria in the suspension was determined as colony forming units (cfu).
Sea bass (Dicentrarchus labrax) weighing an average of 350 g that were used for the implantation work were obtained from a commercial farm in Italy, and were maintained at the Dipartimento di Scienze della Produzione, Universita degli studi di Udine, Udine, Italy. The fish were housed in fibreglass tanks containing seawater of 24‰ salinity at a temperature of 25-26℃.
Cellulose ester dialysis membranes with molecular weight cut-offs of 25 and 300 kDa were obtained from Spectrum Laboratories (USA). They were prepared by boiling in 10 mM NaHCO3, 1 mM EDTA for 30 min then washed twice with sterile distilled water. The membranes were cut into 5 cm pieces, one side was double-sealed with autoclaved string, and the membrane was filled with 1.5 ml of the bacterial suspension. The other end was then double sealed to form a small bag. Care was taken to remove protruding edges, which could injure the fish when the bag was inserted into their abdominal cavity. The 25 kDa bags (n = 8) represented the low molecular weight (LMW) bag and the 300 kDa bags (n = 8) represented the high molecular weight (HMW) bag. When one bag was implanted into a fish, another bag was incubated in vitro in the dark at 22℃. For negative controls, three LMW and HMW bags containing only PBS that had been stored at 22℃ were individually implanted into fish.
Implantation of the dialysis bags was done using a modification of a previously described procedure by Garduño et al. [14]. Fish were anaesthetised with benzocaine (0.06 g/l) and placed on a fish holder with the abdomen up to restrict movement. The skin was disinfected with 70% (v/v) ethanol and a 2 cm long incision, into which the dialysis bag was inserted, taking care not to injure internal organs. The incision was sutured using polyglactin 910 sutures (Ethicon, UK) and the sutured area was treated with dilute iodine solution (250 ppm) to minimise post-surgical infection.
Fish were starved for two days before and after implantation. Then they were fed daily and maintained under the conditions described above for a week. After this time, fish were sacrificed, placed on ice, and taken to the laboratory, where the dialysis bags were removed and fish examined for signs of pasteurellosis. Bacteria from bags of the same pore size were pooled together and placed on ice. The bacterial concentrations within pooled samples were determined as cfu on TSA plates.
A small sample of the bacteria was prepared for electron microscopy and another sample was spread onto a microscope slide, stained with Indian ink, and examined by light microscopy for the presence of a capsule. The remaining bacteria were washed three times with PBS at 2,900 × g at 4℃ for 20 min, and their concentration adjusted to an OD of 1.0 at 610 nm.
The supernatants containing bacterial ECP were also retained. These were filtered through a 0.22 µm filter, precipitated with 40% (w/v) ammonium sulphate overnight at 4℃, and centrifuged at 2,900 × g for 1 h at 4℃. The pellets were washed with 40% (w/v) ammonium sulphate, and dialysed against three changes of PBS using dialysis tubing of the same pore size as that of the implants. The concentration of the ECP preparations was determined using a protein determination kit (BioRad, USA) and adjusted to 100 µg/ml with PBS.
Both bacteria maintained in vivo and in vitro, and their respective ECPs were mixed with electrophoresis sample buffer and stored at -70℃ until analysed.
Bacteria in a 200 µl suspension that had been adjusted to an OD of 1.0 at 610 nm were fixed in Karnovsky's fixative and centrifuged at 2,900 × g for 10 min at 4℃. Fresh fixative was added and the bacteria pelleted as just described. TSA was dropped onto the bacterial pellets and left to solidify. The agar plugs were trimmed and incubated overnight at 4℃ in sodium cacodylate. The sample was postfixed for 1 h with 1% osmium tetroxide, dehydrated through a graded series of ethanol, and embedded in Epoxy resin. Each resin block was incubated at 60℃ for 48 h before obtaining sections approximately 90 nm thick. The sections were counter-stained with uranyl acetate and lead citrate and examined by TEM (HF-3300, Hitachi, Japan).
SDS-PAGE was performed as described previously [18] for whole cell lysates of Ph. d. subsp. piscicida cultured in vivo and in vitro as well as for ECP. Bacteria (1 ml of a suspension with an OD of 1.0 at 610 nm) and ECP (100 mg/ml) that had been previously prepared in sample buffer were boiled for 3 min. A 15 µl volume of each sample was dispensed in individual wells and electrophoresis was conducted at 180 V for 45 min. Pre-stained molecular weight markers (Bio-Rad, USA) were used as standards. The gels were stained with Coomassie brilliant blue R-250 (0.25% w/v) in 50% (v/v) methanol and 10% (v/v) acetic acid for 4 h before destaining.
Bacteria grown in vivo and in vitro, and the ECP of the bacteria grown in vivo were subjected to SDS-PAGE as outlined above, and the separated bacterial components were transferred to a nitrocellulose membrane using 60 V for 70 min. Prestained molecular weight markers (Bio-Rad, USA) were used as standards. Non-specific binding sites on the membrane were blocked with 1% w/v bovine serum albumin in Tris-buffered saline (TBS: 10 mM Tris, 0.5 M NaCl, pH 7.5) for 1 h at 20℃. The membrane was washed three times for 10 min each with TBS containing 0.1% (v/v) Tween-20 (TBST). Membranes were incubated overnight at 4℃ with anti-Ph. d. subsp. piscicida sera raised in sea bass against live Ph. d. subsp. piscicida [18]; the sera from five fish were pooled and diluted 1 : 10 with TBST. Each membrane was washed as described above and then incubated with an anti-sea bass IgM monoclonal antibody (Aquatic Diagnostics, UK) for 3 h at 20℃. Each membrane was then washed three times with TBST before incubation with anti-mouse IgG-biotin conjugate (Diagnostics Scotland, UK) diluted 1 : 100 in TBST. After 2 h at 20℃, each membrane was washed as above and incubated with a 1 : 100 dilution of streptavidin-horseradish peroxide (Diagnostics Scotland, UK) in TBST for a further 2 h at 20℃. The blots were washed three times with TBST, 10 min per wash, once with TBS, and finally with PBS. Each blot was developed in solution containing 20% (v/v) 4-chloro-naphthol (3 mg/ml in methanol) in PBS, to which 0.01% (v/v) hydrogen peroxide was added just before use. The reaction was stopped with distilled water.
Bacteria grown in vivo and in vitro and the ECP recovered from the dialysis bags were subjected to SDS-PAGE as described above. Bacterial components from the gel were transferred to a nitrocellulose membrane, and the total carbohydrate present was determined using an glycoprotein determination kit (Immun-Blot; Bio-Rad, USA) as previously described by Jung et al. [18], with the exception that the membrane was treated with 10 mM sodium periodate in the dark at 22℃ prior to addition of biotinylation solution.
Comparison of bacterial growth in vivo within the dialysis bags to that of bacteria maintained in vitro revealed that scant bacterial growth occurred in dialysis tubing maintained in vitro, while numbers of bacteria raised in vivo were over 1000-fold and 100-fold greater in the LMW and HMW bags, respectively (Table 1). PBS that was used as a negative control in vivo escaped from the implanted bags, as the bags were empty upon sampling.
When bacteria cultured in vivo were examined under a light microscope after staining with Indian ink, they appeared smaller than bacteria grown in vitro and were coccoid in shape. Since it was not possible to determine the presence of capsule in the bacteria produced in vivo using light microscopy, they were examined by TEM.
Ph. d. subsp. piscicida cultured in vivo in the HMW bag (Fig. 1A) and LMW bag (Fig. 1B) possessed a clear dense layer that was not evident with bacteria cultured in vitro in the HMW bag (Fig. 1C). Furthermore, differences were apparent in the size of the bacteria cultured in the LMW bag, which were larger than those raised in the HMW bag or cultured in the bags in vitro. No differences were found between bacteria culture in vitro within the different bags (data not shown).
Differences between bacteria cultured in vivo and in vitro were evident in Coomassie brilliant blue stained SDS-PAGE profiles (Fig. 2). No differences were observed in the molecular weights or the intensity of bands in the SDS-PAGE profiles of bacteria cultured in vitro in the LMW or HMW bags. However, differences were seen between bacteria cultured in vivo in the LMW bag compared with bacteria from the HMW bag. While both populations of bacteria expressed bands with molecular weights of 84, 56, 48, and 18 kDa, a band of approximately 52 kDa was found only with bacteria grown in the HMW bag, and a 45 kDa band was associated with bacteria grown in the LMW bag. Although the latter band was not present in the whole cell lysate of bacteria from the HMW bag, it was present in the ECP of the bacteria cultured in vivo in both bags. The band at 74 kDa in the ECP of bacteria cultured in vivo in both the HMW and LMW bags was particularly heavily stained. Other common bands of the ECP obtained from the two bags included bands at 18, 26, 34, and 45 kDa. There was little similarity in the Coomassie blue profiles between bacteria cultured in vivo and in vitro, although a few common were observed.
Sera collected from fish experimentally infected with Ph. d. subsp. piscicida [19] were used to screen bacteria cultured in vivo and in vitro and the ECP of these bacteria. As shown in Fig. 3, distorted staining of the 74 kDa band was obtained with whole cell extracts of bacteria cultured in vitro and in vivo, and also with ECP from bacteria cultured in vivo. The sera identified bands at 24, 26, 45, 47, and 65 kDa, as well as species of lower molecular weight located at the running front of the gel with bacteria cultured in vitro in both the HMW and LMW bags. Bacteria grown in vivo in the LMW and HMW bags, on the other hand, had no bands in common except for the 74 kDa band. However, a band of approximately 45 kDa was evident in the ECP of bacteria cultured in both the LMW and the HMW bags. Bacteria cultured in vivo in the LMW bag had two bands in common with their ECP (45 and 20 kDa), while bacteria cultured in vivo in the HMW bag did not show any bands in common with ECP except for the 74 kDa band. Bacteria cultured in the HMW bag contained a band at 90 kDa, and a doublet at 18 kDa, while their ECP contained bands at 74 and 45 kDa.
Detection of total carbohydrate revealed only one band at approximately 26 kDa with bacteria cultured in vitro (Fig. 4). Bacteria cultured in the LMW bag in vivo exhibited bands at 26, 27, 29, 34, 68, and 79 kDa, while bacteria cultured in the HMW bag in vivo had bands at 27, 34, 39, and 79 kDa. Staining of the 27 and 79 kDa bands of bacteria cultured in HMW bag in vivo was particularly intense, as were the bands at 26, 27, and 79 kDa of bacteria cultured in the LMW bag in vivo. Carbohydrate staining of the ECP of bacteria grown in vivo was not very clear except for a band at 84 kDa in bacteria cultured in the HMW bag.
In an attempt to examine some of the morphological and antigenic characteristics of Ph. d. subsp. piscicida during infection, the bacterium was cultured in vivo in the peritoneal cavity of European sea bass. Differences in the growth rates were observed between in vivo or in vitro bacterial cultures. The bacteria cultured in vitro showed very little differences in growth because the bacteria were preserved in PBS. Electron microscopy revealed that the bacteria cultured in vivo were smaller and produced a capsular layer, which was more prominent in bacteria cultured in the HMW bag. The smaller appearance of bacteria in vivo may be due to supercoiling, which has been proposed as a mode of regulating virulence genes in response to temperature, anaerobiosis, and osmolarity changes [31]. The capsular layer appeared to be composed of dense material and thin strands projected from the surface of the cell. Elaboration of a capsule by Ph. d. subsp. piscicida appears to depend on the availability of iron and the growth phase of the bacterium, with iron limitation and an early growth phase inducing a distinct capsule [10]. The presence of a capsular layer on the bacterium confers resistance to serum killing and increases the degree of virulence of encapsulated strains [2,10].
The present observation of fewer protein bands in in vivo grown bacteria as compared to their in vitro grown counterparts may be a consequence of the different exclusion limits of the dialysis bags, variations in size and holding conditions of the fish, and the use of different isolates. Sea bass sera raised against Ph. d. subsp. piscicida recognised antigens of differing molecular weights depending on whether the bacteria had been cultured in vitro or in vivo. The absence of low molecular weight antigens in bacteria cultured in vivo agrees with previous observations [3,4]. Bakopoulos et al. [4] identified bands at 52, 44, and 21.3 kDa using sea bass sera raised against live bacteria, and bands at 52, 44, 39.5, 34.7, and 21.3 kDa with sea bass sera raised against formalin inactivated cells cultured in a modified yeast based medium. The 45 and 20 kDa bands from bacteria grown in vivo in the present study may represent the 21.3 and 44 kDa bands identified by Bakopoulos et al. [4]. However the bands recognized on bacteria from the HMW bag in this study were not reported by Bakopoulos et al. [4], presumably because these authors used dialysis tubing with a low MWCO, thus restricting the flow of nutrients through the bag.
The effect of iron limitation on the antigenicity of the bacterium using sera from sea bass injected with live and heat killed Ph. d. subsp. Piscicida to screen bacteria cultured under iron limitation has been previously reported by Jung et al. [19]. Two major bands at 24 and 47 kDa were recognized by the sera when bacteria were cultured in TSB, and at 45 kDa and 22 kDa when bacteria were cultured in iron restricted TSB. In the previous and present studies, sera raised against live bacteria reacted with bands of 24, 22, 20, and 18 kDa depending on whether the bacteria had been cultured in TSA, under iron limitation, or in vivo. The variations observed in the antigenic bands could have been caused due to differential expression of these antigens by Ph. d. subsp. piscicida depending on its culture environment. Alternatively, the bands may represent different molecules that share the same antigenic site, or the same molecule may be processed differently by the immune system of the fish. The bands at 47 (present under TSB culture) and 45 kDa (present under iron limitation and culture in vivo) are also thought to be the same antigen, which differ in size depending on the growth environment of the bacterium. This band is also present in the ECP of bacteria cultured in the LMW bag, suggesting that this molecule is secreted.
Differences observed in the glycoprotein profile of the bacteria cultured in vitro and in vivo suggests that the expression of these protein molecules is dependent on bacterial culture conditions. The carbohydrate bands identified in the present study may represent sialic acids [18]. High molecular weight carbohydrate material was also present on the bacteria, perhaps related to the presence of a bacterial capsule in vivo.
The overall variations observed between bacteria cultured in vivo and in vitro may reflect differences in their virulence or in antigen expression. When A. salmonicida is grown in vivo in the peritoneal cavity of rainbow trout a capsule is produced, which is linked to virulence [14]. Aeromonas salmonicida grown in glucose-rich medium (GRM) in vitro also produce capsular material [13]. Thornton et al. [32] reported that when A. salmonicida are grown within the peritoneal cavity of rainbow trout, unique antigens are expressed as determined by Western blot analysis using immune rabbit serum raised against cells grown in vivo. Comparison between bacteria cultured in GRM and in vivo by Western blots conducted using this serum have shown that bacteria cultured in GRM possess similar surface antigens to those expressed in vivo [14]. The ECP of Ph. d. subsp. piscicida cultured in vivo appears more toxic than ECP of bacteria grown in vitro [4].
It was presently noted that after removing the bags from the peritoneal cavity of the fish, most bags were covered with a thin layer of cells. Histological examination of these dialysis bags revealed that the bags were covered with fibrocytes and fibroblasts, together with a small amount of inflammatory cells (data not shown). When this layer was first observed, it was assumed that macrophage infiltration had occurred, as inflammatory cells are known to migrate to sites of bacterial stimulus [9]. However, the cells were identified as fibroblasts and fibrocytes, and very few macrophages were present. The nature of the cells covering the bags suggests that the ECP of Ph. d. subsp. piscicida does not trigger an inflammatory response in the peritoneal cavity, or the ECP produced by the bacteria are unable to pass through the membrane of the bags.
The differences seen between bacteria cultured within the HMW and the LMW bags in vivo may be a result of differences in nutrients and soluble substances present within the two bags or may represent different stages of infection. The immune system of fish may recognise different antigens associated with phenotypic changes in the bacterium due to culture conditions, and this may explain differences seen in the molecular weight of some molecules recognised by the fish antibodies (i.e. the 24, 22, 20, and 18 kDa bands). Further detailed studies on the identification of the antigenic bands and their importance in pathogenicity of the bacterium and efficiency for inducing an immune response will be valuable in spurring the development of a vaccine against pasteurellosis.
Figures and Tables
Fig. 1
Electron microscopic examination of Photobacterium damselae subsp. piscicida (I752) cultured in vivo and in vitro. Arrows indicate capsular material. (A) Bacteria grown in vivo in 300 kDa dialysis tubing (HMW bag). Bar = 0.1 µm. (B) Bacteria grown in vivo in 25 kDa dialysis tubing (LMW bag). Bar = 0.1 µm. (C) Bacteria grown in vitro in 300 kDa dialysis tubing (HMW bag). Bar = 0.2 µm.

Fig. 2
Coomassie brilliant staining of SDS-PAGE of Photobacterium damselae subsp. piscicida and their extracellular products (ECP) after culturing in vivo and in vitro. Lane 1: molecular weight marker, Lane 2: bacteria maintained in vitro in 25 kDa dialysis tubing (LMW bag), Lane 3: concentrated ECP of bacteria shown in Lane 2, Lane 4: bacteria maintained in vitro in 300 kDa dialysis tubing (HMW bag), Lane 5: concentrated ECP of bacteria shown in Lane 4, Lane 6: bacteria maintained in vivo in 25 kDa dialysis tubing (LMW bag), Lane 7: concentrated ECP of bacteria shown in Lane 6, Lane 8: bacteria maintained in vivo in 300 kDa dialysis tubing (HMW bag), Lane 9: concentrated ECP of bacteria shown in Lane 8.
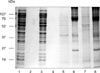
Fig. 3
Western blot analysis with anti-Photobacterium damselae subsp. piscicida sea bass sera against Ph. d. subsp. piscicida (I752) and their extracellular products (ECP) after culture in vivo and in vitro. Lane 1: bacteria maintained in vitro in 25 kDa dialysis tubing (LMW bag), Lane 2: concentrated ECP of bacteria shown in Lane 1, Lane 3: bacteria maintained in vitro in 300 kDa dialysis tubing (HMW bag), Lane 4: concentrated ECP of bacteria shown in Lane 3, Lane 5: bacteria maintained in vivo in 25 kDa dialysis tubing (LMW bag), Lane 6: concentrated ECP of bacteria shown in Lane 5, Lane 7: bacteria maintained in vivo in 300 kDa dialysis tubing (HMW bag), Lane 8: concentrated ECP of bacteria shown in Lane 7.
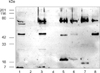
Fig. 4
Carbohydrate determination on Photobacterium damselae subsp. piscicida (I752) and in their extracellular products (ECP) after culture in vivo and in vitro. Lane 1: concentrated ECP of bacteria shown in Lane 2, Lane 2: bacteria maintained in vivo in 300 kDa dialysis tubing (HMW bag), Lane 3: concentrated ECP of bacteria shown in Lane 4, Lane 4: bacteria maintained in vivo in 25 kDa dialysis tubing (LMW bag), Lane 5: concentrated ECP of bacteria shown in Lane 6, Lane 6: bacteria maintained in vitro in 300 kDa dialysis tubing (HMW bag), Lane 7: concentrated ECP of bacteria shown in Lane 8, Lane 8: bacteria maintained in vitro in 25 kDa dialysis tubing (LMW bag).
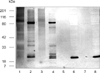
Table 1
Growth of Photobacterium damselae subsp. piscicida in vivo and in vitro in dialysis tubing bags
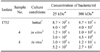
*Pore size of dialysis bag. †Initial concentration of bacterial suspension added to the dialysis tubing bags. §Dialysis tubing bags maintained in an incubator at 22℃ for 7 days. ‡Dialysis tubing bags Implanted into the peritoneal cavity of sea bass for 7 days. The in vivo and in vitro negative control bags containing PBS did not show any bacterial growth. Data are presented as mean ± SD.
Acknowledgments
The authors would like to thank Mr. Marco Zanini and Dr. Rodolfo Ballestrazzi (Dipartimento di Scienze della Produzione Animale, Universita di Udine, Italy) and Ms. Ana Ines Rivas Salas (Institute of Aquaculture, University of Stirling, Stirling, UK).
References
1. Arijo S, Balebona C, Martinez-Manzanares E, Moriñigo MA. Immune response of gilt-head seabream (Sparus aurata) to antigens from Photobacterium damselae subsp. piscicida. Fish Shellfish Immunol. 2004. 16:65–70.

2. Arijo S, Borrego JJ, Zorrilla I, Balebona MC, Moriñigo MA. Role of the capsule of Photobacterium damsela subsp. piscicida in protection against phagocytosis and killing by gilt-head seabream (Sparus aurata, L) macrophages. Fish Shellfish Immunol. 1998. 8:63–72.

3. Bakopoulos V, Adams A, Richards RH. The effect of iron limitation growth conditions on the cell and extracellular components of the fish pathogen Pasteurella piscicida. J Fish Dis. 1997. 20:297–305.

4. Bakopoulos V, Hanif A, Poulos K, Galeotti M, Adams A, Dimitriadis GJ. The effect of in vivo growth on the cellular and extracellular components of the marine bacterial pathogen Photobacterium damsela subsp. piscicida. J Fish Dis. 2004. 27:1–14.
5. Bakopoulos V, Pearson M, Volpatti D, Gousmani L, Adams A, Galeotti M, Dimitriadis GJ. Investigation of media formulations promoting differential antigen expression by Photobacterium damsela ssp. piscicida and recognition by sea bass, Dicentrarchus labrax (L.), immune sera. J Fish Dis. 2003. 26:1–13.

6. Bakopoulos V, Volpatti D, Gusmani L, Galeotti M, Adams A, Dimitriadis GJ. Vaccination trials of sea bass, Dicentrarchus labrax (L.), against Photobacterium damsela subsp. piscicida, using novel vaccine mixtures. J Fish Dis. 2003. 26:77–90.

7. Bonet R, Magariños B, Romalde JL, Simon-Pujol MD, Toranzo AE, Congregado F. Capsular polysaccharide expressed by Pasteurella piscicida grown in vitro. FEMS Microbiol Lett. 1994. 124:285–289.

8. Brown MR, Anwar H, Costerton JW. Surface antigens in vivo: a mirror for vaccine development. Can J Microbiol. 1988. 34:494–498.
9. do Vale A, Afonso A, Silva MT. The professional phagocytes of sea bass (Dicentrarchus labrax L.): cytochemical characterisation of neutrophils and macrophages in the normal and inflamed peritoneal cavity. Fish Shellfish Immunol. 2002. 13:183–198.

10. do Vale A, Ellis AE, Silva MT. Electron microscopic evidence that expression of capsular polysaccharide by Photobacterium damselae subsp. piscicida is dependent on iron availability and growth phase. Dis Aquat Organ. 2001. 44:237–240.

11. do Vale A, Magariños B, Romalde JL, Lemos ML, Ellis AE, Toranzo AE. Binding of haemin by the fish pathogen Photobacterium damselae subsp. piscicida. Dis Aquat Organ. 2002. 48:109–115.

12. Díaz-Rosales P, Chabrillón M, Moriñigo MA, Balebona MC. Survival against exogenous hydrogen peroxide of Photobacterium damselae subsp. piscicida under different culture conditions. J Fish Dis. 2003. 26:305–308.
13. Garduño RA, Kay WW. Capsulated cells of Aeromonas salmonicida grown in vitro have different functional properties than capsulated cells grown in vivo. Can J Microbiol. 1995. 41:941–945.

14. Garduño RA, Thornton JC, Kay WW. Aeromonas salmonicida grown in vivo. Infect Immunol. 1993. 61:3854–3862.
15. Garrote A, Bonet R, Merino S, Simon-Pujol MD, Congregado F. Occurrence of a capsule in Aeromonas salmonicida. FEMS Microbiol Lett. 1992. 95:127–132.
16. Hawke JP, Thune RL, Cooper RK, Judice E, Kelly-Smith M. Molecular and phenotypic characterization of strains of Photobacterium damselae subsp. piscicida isolated from hybrid striped bass cultured in Louisiana, USA. J Aquat Anim Health. 2003. 15:189–201.

17. Juiz-Río S, Osorio CR, Lemos ML. Identification and characterisation of the fur genes in Photobacterium damselae ssp. piscicida and ssp damselae. Dis Aquat Organ. 2004. 58:151–156.
18. Jung TS, Thompson KD, Adams A. A comparison of sialic acid between different isolates of Photobacterium damselae subsp. piscicida. Fish Pathol. 2001. 36:217–224.

19. Jung TS, Thompson KD, Volpatti D, Galeotti M, Adams A. Variation in the molecular weight of Photobacterium damselae subsp. piscicida antigens when cultured under different conditions in vitro. J Vet Sci. 2007. 8:255–261.

20. Kawakami H, Sakai M. Comparison of susceptibility of seven fishes to Photobacterium damsela subsp. piscicida. Bull Eur Assoc Fish Pathol. 1999. 19:153–155.
21. Kawakami H, Shinohara N, Fukuda Y, Yamashita H, Kihara H, Sakai M. The efficacy of lipopolysaccharide mixed chloroform-killed cell (LPS-CKC) bacterin of Pasteurella piscicida on Yellowtail, Seriola quinqueradiata. Aquaculture. 1997. 154:95–105.

22. Kawakami H, Shinohara N, Sakai M. The non-specific immunostimulation and adjuvant effects of Vibrio anguillarum bacterin, M-glucan, chitin and Freund's complete adjuvant against Pasteurella piscicida infection in yellowtail. Fish Pathol. 1998. 33:287–292.

23. Magariños B, Romalde JL, Lemos ML, Barja JL, Toranzo AE. Iron uptake by Pasteurella piscicida and its role in pathogenicity for fish. Appl Environ Microbiol. 1994. 60:2990–2998.

24. Magariños B, Romalde JL, Lopez-Romalde S, Morinigo MA, Toranzo AE. Pathobiological characterization of Photobacterium damselae subsp. piscicida strains isolated from cultured sole (Solea senegalensis). Bull Eur Assoc Fish Pathol. 2003. 23:183–190.
25. Magariños B, Romalde JL, Santos Y, Casal JF, Barja JL, Toranzo AE. Vaccination trials on gilt-head sea bream (Sparus aurata) against Pasteurella piscicida. Aquaculture. 1994. 120:201–208.

26. Magariños B, Santos Y, Romalde JL, Rivas C, Barja JL, Toranzo AE. Pathogenic activities of live cells and extracellular products of the fish pathogen Pasteurella piscicida. J Gen Microbiol. 1992. 138:2491–2498.
27. Moriñigo MA, Romalde JL, Chabrillon M, Magariños B, Arijo S, Balebona MC, Toranzo AE. Effectiveness of a divalent vaccine for gilthead sea bream (Sparus aurata) against Vibrio alginolyticus and Photobacterium damselae subsp. piscicida. Bull Eur Assoc Fish Pathol. 2002. 22:298–303.
28. Nitzan S, Shwartsburd B, Heller ED. The effect of growth medium salinity of Photobacterium damselae subsp. piscicida on the immune response of hybrid bass (Morone saxatilis × M. chrysops). Fish Shellfish Immunol. 2004. 16:107–116.

29. Nitzan S, Shwartsburd B, Vaiman R, Heller ED. Some characteristics of Photobacterium damselae ssp. piscicida isolated in Israel during outbreaks of pasteurellosis in hybrid bass (Morone saxatilis × M. chrysops). Bull Eur Assoc Fish Pathol. 2001. 21:77–80.
30. Romalde JL. Photobacterium damselae subsp. piscicida: an integrated view of a bacterial fish pathogen. Int Microbiol. 2002. 5:3–9.

31. Salyers AA, Whitt DD. Bacterial Pathogenesis: A Molecular Approach. 1994. 1st ed. Washington DC: ASM Press;63–72.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download