Abstract
Piroplasms are tick-transmitted, intracellular, hemoprotozoan parasites that cause anorexia, fever, anemia, and icterus. Theileriosis is caused by Theileria sergenti and causes major economic losses in grazing cattle in Japan and Korea. In May 2003, we examined the antigenic diversity of the major piroplasm surface protein (MPSP) gene in 35 healthy Jeju black cattle that were born and raised at the National Institute of Subtropical Agriculture. On microscopic examination of Giemsa-stained blood smears, 9 of 35 cattle had intra-erythrocytic piroplasms. Hematological data were within normal range for all 35 cattle. Amplification of DNA from all blood samples using universal MPSP gene primers showed mixed infections with C, I, and B type Theileria spp. Type C was identified in 20 of 35 blood samples, and type B was identified in 17 samples. Allelic variation was seen in type B.
Theileria spp. are tick-transmitted, intracellular, hemoprotozoan parasites that cause anorexia, fever, anemia, and icterus. Bovine theileriosis caused by T. sergenti is a major source of economic losses in grazing cattle in Japan and Korea. In Korea, bovine piroplasmosis is caused by T. sergenti [1,6] and B. ovata [2]. Infected cattle suffer from chronic anemia owing to intra-erythrocytic piroplasms and occasionally die in severe cases. After the acute phase, the infection may follow a chronic, subclinical course, and animals can become piroplasm carriers, acting as reservoirs.
Major piroplasm surface protein (MPSP) is a major target antigen recognized by the host immune system; it shows antigenic polymorphism as an immunity evasion mechanism [10,22]. Non-pathogenic Theileria spp. are divided into at least five types based on alleles of the MPSP gene: I (Ikeda), C (Chitose), B (Buffeli) 1 and 2, and Thai types [3,8,10,12,13,18]. Field isolates from Japan, Korea, Australia, and other Asian and European countries are reported to contain mixed populations of parasites bearing various combinations of the MPSP allele [3,5,10,18,20,21]. In Japan, Theileria spp. consist of type I, C, and B2 parasites [10,12]. In Korea, type I is common, and co-infection with types I and C is known to occur. Some Korean isolates include parasites with the B1 allele, which is seen only in T. orientalis/buffeli. This suggests that T. orientalis/buffeli co-exists with T. sergenti in Korea [12].
In this study, we examined the antigenic diversity of the Theileria MPSP gene in Jeju black cattle.
In May 2003, blood samples were collected from 35 Jeju black cattle at the National Institute of Subtropical Agriculture (Jeju, Korea), placed in EDTA tubes, and stored at -70℃ until DNA extraction. To evaluate intracellular parasites, thin blood film smears were made from fresh blood and stained with Giemsa using standard methods.
DNA was extracted from frozen blood samples using a modification of Miller's method [15]. For each sample, 500 µl of blood was mixed with two volumes of STE buffer (10 mM Tris-HCl, pH 8.0, 1 mM EDTA, 0.1 M NaCl) and then centrifuged at 12,000 × g for 5 min. The pellets were washed two or three times in STE buffer, and the cellular debris was removed after each wash. The pellets were resuspended in SDS-proteinase K buffer (0.1 mg/µl) and incubated at 37℃ overnight. The DNA was extracted with phenol-chloroform-isoamyl alcohol (25 : 24 : 1 by vol; Sigma, USA). The samples were then extracted with chloroform-isoamyl alcohol (24 : 1 by vol; Sigma, USA), and the DNA was precipitated with cold ethanol. The DNA pellet was resuspended in 100 µl of dH2O.
Four sets of primers were used. The first pair, Ts-U and Ts-R (875 bp), are universal primers for the Theileria MPSP gene [11]. Different sense primers-Ts-C (831 bp) [13], Ts-I (826 bp) [8], and Ts-B (826 bp) [10]-were used together with Ts-R to amplify the MPSP genes of T. sergenti (types C and I) and T. buffeli (type B), respectively. The amplification mixture contained 10 × PCR buffer, 20 pmol of each primer, one unit of Taq polymerase (Takara, Japan), 200 mM of each dNTP, and 50-100 ng of template DNA in a final volume of 20 µl.
PCR was performed in the TaKaRa PCR thermal cycler (Takara Shuzo, Japan) with an initial denaturation of 94℃ for 5 min, followed by 35-40 cycles of 1 min at 94℃, 30 sec at 57℃, and 1 min at 72℃, and a final extension at 72℃ for 7 min. The sizes of the PCR products were estimated through co-electrophoresis of 5 µl of the reaction mix and a 100-bp ladder in 1.2% agarose gels (Sea Kem; FMC Bioproducts, USA), which were visualized by UV transillumination of the ethidium bromide-stained DNA. The amplified products of primers Ts-B and Ts-R were analyzed using RFLP, as described previously [7,18], to distinguish types B1 and B2. The PCR products were digested with restriction enzymes BglI (Bioneer, Korea), DraI (Takara, Japan), EcoT14I (Bioneer, Korea), EcoRV (Bioneer, Korea), and HindIII (Takara, Japan). Each reaction mixture contained 1 µl of PCR product, 1 µl of buffer (×10), 10-15 units of restriction enzyme, and dH2O to a final volume of 10 µl. The reaction mixture was incubated at 37℃ for 2 h. The sizes of the digested PCR products were estimated through co-electrophoresis of 5 µl of the reaction mix with a standard size marker (HaeIII-digested ØX174) in 2% agarose gels (Sea Kem; FMC Bioproducts, USA), which were visualized by UV transillumination of the ethidium bromide-stained DNA.
The PCR products were electrophoresed in a 1.2% agarose gel, and the band of the correct size was excised. The B-type amplicons were recovered from the agarose gel using a DNA gel extraction kit (Geneclean 11 Kit; Q-Bio Gene, USA), according to the manufacturer's instructions. The fragments were cloned using the pGEM-T easy vector system (Promega, USA) and transformed into DH5 One Shot Escherichia coli, according to the manufacturer's instructions. An AccuPrep Plasmid Extraction kit (Bioneer, Korea) was used to isolate the cloned DNA. The presence of an insert was verified using primers T7 and Ts-R. Two clones were chosen for sequencing. The MPSP gene sequences determined in this study were compared with the T. sergenti (accession number: D50304, D11046, AB016280), T. buffeli (D11047), and T. orientalis (AB008369) sequences in GenBank. The sequences were aligned and analyzed using the Clustal V method in MegAlign software (DNA Star, USA). The phylogenetic tree was constructed using the DNASTAR program, with B. equi as an out-group. The GenBank accession numbers for the sequences used in the analysis were as follows: T. sergenti-D50304 (Aomori), D11046 (Ikeda), AB016280 (Fukushima); T. buffeli-D11047 (Warwick); T. orientalis-AB008369 (Essex); B. equi-L13784; Jeju black cattle (JBC)-1, 2-Theileria isolate from Jeju black cattle.
The hematological values of all the samples were within the normal range (data not shown). On microscopic examination of Giemsa-stained blood smears, 9 of 35 cattle had intra-erythrocytic piroplasms. The mean packed cell volumes were 40 ± 5.9% in the 9 parasitemic cows and 37 ± 5.2% in the 26 non-parasitemic cows.
The universal Theileria MPSP primers amplified an 875-bp fragment from all of the blood samples. The different sense primers amplified the different MPSP alleles: Ts-C, Ts-I, and Ts-B amplified types C (831 bp), I (826 bp), and B (826 bp), respectively (Table 1). (Table 2)
Allele-specific PCR identified mixed infections with types C, I, and B. Type C was identified in 20 of 35 blood samples, and type B was identified in 17 samples. Eleven samples contained unknown types (Table 3).
When the products amplified using primers Ts-B and Ts-R were analyzed using RFLP, 11 of 17 amplicons produced the B-type pattern shown in Fig. 1A. Three restriction enzymes-BglI, DraI, and EcoT14I-lacked enzyme sites in these 11 amplicons. By contrast, EcoRV and HindIII digested the PCR products and produced three and four bands, respectively. Five of the 17 amplicons resulted in patterns similar to Fig. 1A through DraI, EcoT14I, EcoRV, and HindIII. BglI produced two bands (Fig. 1B). In the remaining amplicon, one of the B types showed variation in the HindIII site, producing two bands, as shown in Fig. 1C.
The two sequences obtained in this study were compared with five MPSP sequences for Theileria spp. reported in GenBank. The results are shown in Fig. 2. The two sequences of Theileria spp. isolated from Jeju black cattle showed 88% (JBC-1) and 90% (JBC-2) homology with type B2 (D50304) and 95% (JBC-1) and 88% (JBC-2) homology with type B1 (D11047). In the phylogenetic tree, the two sequences of Theileria spp. isolated from Jeju black cattle were related to T. orientalis (Essex) and T. buffeli (Warwick) (Fig. 3).
The major clinical sign of bovine piroplasmosis is hemolytic anemia, but this sign may not be obvious in herds with subclinical infections [20]. A combination of predisposing factors influences the course of the clinical illness. Although we found piroplasms in nine cows on microscopic examination, all blood samples were positive for Theileria spp. by PCR, and all cows had subclinical infections.
The prevalence of T. sergenti infection in Jeju [9] was higher than that seen in other provinces [19]. The major biological vector of T. sergenti in Korea and Japan, Haemaphysalis longicornis, has also been shown to transmit B. ovata [2] and B. caballi experimentally [16]. Some investigators have suggested that the presence of multiple parasite clones in a vector is essential for cross-fertilization, which increases genetic diversity [12,15].
The majority of T. sergenti-infected cattle in Japan contain a mixed population of type I and C parasites [8,13]. T. buffeli is distributed mainly in Australia and adjacent areas in Asia [5,12,21]. In Taiwan and other parts of East Asia, the type I parasite has not been identified [3,17,21], while type I is the major parasite in Japan and Korea [5,9,12]. The relationship between the allelic form and the virulence of T. sergenti/buffeli is not clear, though there is evidence to suggest that type I is more pathogenic than types C and B. In Korea, Ikeda (type I) stock is more pathogenic than Fukushima (type C) stock; in a previous study, all Theileria isolates were type I, and the cattle exhibited severe symptoms [5]. In our study, type I was rare (6 of 35); most of the isolates were types C (20 of 35) and B (17 of 35), and all cattle were normal clinically and on hematological examination.
In this study, we used PCR-RLFP to subclassify type B, as described previously [5,12]. The major pattern identified was type B1 (11 of 17), and 5 of the 17 isolates were a mix of types B1 and B2. One sample exhibited a new pattern, with variation at a HindIII site. Sequence analysis confirmed the similarity between the MPSP gene and type B. The results of the sequence and phylogenetic analyses suggest that the isolate from Jeju black cattle is closely related to T. sergenti (type B2) and T. buffeli (type B1), although this is based on a comparison of only part of the MPSP gene [7]. The MPSP gene should be sequenced completely to allow comparison with samples isolated from other countries.
Kubota et al. [12] demonstrated that the ratio of type I and C parasites in the population changes during persistent infection in cattle. Iwasaki et al. [4] provided further evidence of a population shift from parasites expressing one MPSP allele to those expressing another, resulting in an apparent change in parasite antigenicity.
Many studies have reported that the susceptibility to piroplasmosis differs with breed. Kim et al. [9] reported that Korean native cattle are more resistant to T. sergenti infection than are Holsteins in Jeju. Our results suggest that the differential resistance is based on the breed and host immune response. Further studies of the resistance and adaptation of Jeju black cattle in Jeju compared with other breeds are necessary.
This study identified mixed infections of Theileria spp. based on MPSP alleles. In addition, there are allelic variants in Jeju. Therefore, further studies of the tick vector, the antigenic difference between variants of each type, and the seasonal variation in allele type are essential for developing optimal treatment and control methods.
Figures and Tables
Fig. 1
Restriction pattern of the PCR product amplified with primers Ts-B and Ts-R. The PCR product was digested with restriction enzymes BglI (Bg), DraI (D), EcoT14I (E14), EcoRV (EV), and HindIII (H), electrophoresed on a 2.0% agarose gel, and stained with ethidium bromide. Lane M: marker (HaeIII digested ØX174), U (undigested): PCR product. A: B-type pattern; B, C: B-type similar pattern.
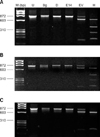
Fig. 2
Comparison of the partial nucleotide sequences of the PCR product from Jeju black cattle (JBC-1, 2) and MPSP genes of other Theileria spp. from the GenBank database. Gaps (-) indicate spaces introduced into the aligned sequences by the multiple alignment program in CLUSTAL W. An asterisk represents identical nucleotides. T. sergenti-D50304 (Aomori), D11046 (Ikeda), AB016280 (Fukushima); T. buffeli-D11047 (Warwick); T. orientalis-AB008369 (Essex); Jeju black cattle (JBC)-1, 2 - Theileria isolate from Jeju black cattle.

Fig. 3
Phylogenetic tree for the MPSP gene of Theileria parasites. This phylogenetic tree was constructed using the DNASTAR program, with B. equi as an out-group. The GenBank accession numbers for the sequences used in the analysis are as follows: T. sergenti-D50304 (Aomori), D11046 (Ikeda), AB016280 (Fukushima); T. buffeli-D11047 (Warwick); T. orientalis-AB008369 (Essex); B. equi-L13784; JBC-1, 2 - Theileria isolate from Jeju black cattle.

References
1. Chae JS, Lee JM, Kwon OD, Lee SO, Chae KS, Onuma M. Comparative analyses of Theileria sergenti isolated from Korea and Japan by Southern hybridization and polymerase chain reaction. Korean J Vet Res. 1996. 36:187–193.
2. Cho SH, Kim TS, Lee HW, Tsuji M, Ishihara C, Kim JT, Wee SH, Lee CG. Identification of newly isolated Babesia parasites from cattle in Korea by using the Bo-RBC-SCID mice. Korean J Parasitol. 2002. 40:33–40.

3. Inoue M, Van Nguyen D, Meas S, Ohashi K, Sen S, Sugimoto C, Onuma M. Survey of Theileria parasite infection in cattle in Cambodia and Vietnam using piroplasm surface protein gene-specific polymerase chain reaction. J Vet Med Sci. 2001. 63:1155–1157.

4. Iwasaki T, Kakuda T, Sako Y, Sugimoto C, Onuma M. Differentiation and quantification of Theileria sergenti piroplasm types using type-specific monoclonal antibodies. J Vet Med Sci. 1998. 60:665–669.

5. Kakuda T, Kubota S, Sugimoto C, Baek BK, Yin H, Onuma M. Analysis of immunodominant piroplasm surface protein genes of benign Theileria parasites distributed in China and Korea by allele-specific polymerase chain reaction. J Vet Med Sci. 1998. 60:237–239.

6. Kang SW, Choi EJ, Kweon CH. Cloning and sequencing of p33 in a Korean isolate of Theileria sergenti. Korean J Parasitol. 1997. 35:105–110.

7. Katzer F, McKellar S, Kirvar E, Shiels B. Phylogenetic analysis of Theileria and Babesia equi in relation to the establishment of parasite populations within novel host species and the development of diagnostic tests. Mol Biochem Parasitol. 1998. 95:33–44.

8. Kawazu S, Sugimoto C, Kamio T, Fujisaki K. Analysis of the genes encoding immunodominant piroplasm surface proteins of Theileria sergenti and Theileria buffeli by nucleotide sequencing and polymerase chain reaction. Mol Biochem Parasitol. 1992. 56:169–175.

9. Kim GH, Lee KK, Onuma M. Susceptibility of Theileria sergenti Infection in Holstein Cattle Compared to Korean Native Cattle on Cheju Island. J Protozool Res. 1999. 9:103–112.
10. Kubota S, Sugimoto C, Kakuda T, Onuma M. Analysis of immunodominant piroplasm surface antigen alleles in mixed populations of Theileria sergenti and T. buffeli. Int J Parasitol. 1996. 26:741–747.

11. Kubota S, Sugimoto C, Onuma M. A genetic analysis of mixed population in Theileria sergenti stocks and isolates using allele-specific polymerase chain reaction. J Vet Med Sci. 1995. 57:279–282.

12. Kubota S, Sugimoto C, Onuma M. Population dynamics of Theileria sergenti in persistently infected cattle and vector ticks analysed by a polymerase chain reaction. Parasitology. 1996. 112:437–442.

13. Matsuba T, Kubota H, Tanaka M, Hattori M, Murata M, Sugimoto C, Onuma M. Analysis of mixed parasite populations of Theileria sergenti using cDNA probes encoding a major piroplasm surface protein. Parasitology. 1993. 107:369–377.

14. Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988. 16:1215.

15. Onuma M, Kakuda T, Sugimoto C. Theileria parasite infection in East Asia and control of the disease. Comp Immunol Microbiol Infect Dis. 1998. 21:165–177.
16. Rodríguez Bautista JL, Ikadai H, You M, Battsetseg B, Igarashi I, Nagasawa H, Fujisaki K. Molecular evidence of Babesia caballi (Nuttall and Strickland, 1910) parasite transmission from experimentally infected SCID mice to the ixodid tick, Haemaphysalis longicornis (Neuman, 1901). Vet Parasitol. 2001. 102:185–191.
17. Sarataphan N, Kakuda T, Chansiri K, Onuma M. Survey of benign Theileria parasites of cattle and buffaloes in Thailand using allele-specific polymerase chain reaction of major piroplasm surface protein gene. J Vet Med Sci. 2003. 65:133–135.

18. Sarataphan N, Nilwarangkoon S, Tananyutthawongese C, Kakuda T, Onuma M, Chansiri K. Genetic diversity of major piroplasm surface protein genes and their allelic variants of Theileria parasites in Thai cattle. J Vet Med Sci. 1999. 61:991–994.

19. Song KH, Sang BC. Prevalence of Theileria sergenti infection in Korean native cattle by polymerase chain reaction. Korean J Parasitol. 2003. 41:141–145.

20. Stockham SL, Kjemtrup AM, Conrad PA, Schmidt DA, Scott MA, Robinson TW, Tyler JW, Johnson GC, Carson CA, Cuddihee P. Theileriosis in a Missouri beef herd caused by Theileria buffeli: case report, herd investigation, ultrastructure, phylogenetic analysis, and experimental transmission. Vet Pathol. 2000. 37:11–21.

21. Wang CT, Kubota S, Kakuda T, Kuo CC, Hsu TL, Onuma M. Survey of Theileria parasite infection in cattle in Taiwan. J Vet Med Sci. 1998. 60:253–255.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download