Abstract
Bovine tuberculosis (TB) is a major zoonosis that's caused by Mycobacterium bovis (M. bovis). Being able to detect M. bovis is important to control bovine TB. We applied a molecular technique, the variable number tandem repeat (VNTR) typing method, to identify and distinguish the M. bovis isolates from Gyeonggi-do, Korea. From 2003 to 2004, 59 M. bovis clinical strains were isolated from dairy cattle in Gyeonggi-do, Korea, and these cattle had tuberculosis-like lesions. Twenty-four published MIRU-VNTR markers were applied to the M. bovis isolates and ten of them showed allelic diversity. The most discriminatory locus for the M. bovis isolates in Korea was QUB 3336 (h = 0.64). QUB 26 and MIRU 31 also showed high discriminative power (h = 0.35). The allelic diversity by the combination of all VNTR loci was 0.86. Six loci (MIRU 31, ETR-A and QUB-18, -26, -3232, -3336) displayed valuable allelic diversity. Twelve genotypes were identified from the 59 M. bovis isolates that originated from 20 cattle farms that were dispersed throughout the region of Gyenggi-do. Two genotypes [designation index (d.i.) = e, g] showed the highest prevalence (20% of the total farms). For the multiple outbreaks on three farms, two successive outbreaks were caused by the same genotype at two farms. Interestingly, the third outbreak at one farm was caused by both a new genotype and a previous genotype. In conclusion, this study suggests that MIRU-VNTR typing is useful to identify and distinguish the M. bovis isolates from Gyeonggi-do, Korea.
Mycobacterium bovis (M. bovis) is the cause of bovine tuberculosis (TB), which is a major zoonosis. M. bovis has a broad range of hosts that includes humans [12]. Bovine TB affects more than 500 dairy cattle each year in Korea and it is responsible for major agricultural economic losses. Bovine TB especially affects people in the developing countries. In some of these countries, M. bovis is responsible for 5-10% of all human TB and 30% of all the child TB patients [26].
Diagnosis is important to control bovine TB and to help block transmission not only to animals, but also to humans. To control bovine TB, it is necessary to know how many and which M. bovis strains are dispersed in the field. Classical bacteriological methods are important for isolating pathogenic bacteria from the samples, but these methods are unable to distinguish strains among the same species. The advent of molecular techniques has greatly contributed to the identification and typing of M. bovis [13]. The molecular techniques also enable identification of M. bovis in a short time because M. bovis requires 3-4 weeks to grow [8]. Moreover, the molecular typing method can distinguish M. bovis from other M. tuberculosis complex and it can discriminate between clinical M. bovis isolates. This epidemiological information is useful for tracing the outbreaks and transmission among domestic or wild animals [24,25]. Molecular typing methods such as restriction fragment length polymorphism (RFLP), spoligotyping and variable number tandem repeat (VNTR) analysis have been used. One RFLP method is IS6110 RFLP, which analyzes the polymorphism of the insertion sequence 6110 (IS6110), which is the typical repetitive sequence of the Mycobacterium tuberculosis complex. IS6110 RFLP has shown high discriminatory power and sensitivity and it has been widely used for molecular typing of the M. tuberculosis complex [2,14,23]. However, the IS6110 RFLP method is not applicable to the typing of M. bovis because M. bovis has only a single or a few IS6110 copies [6].
VNTR typing is a PCR-based typing method that analyzes the variations in the number of tandem repeated sequences that are distributed across several loci of the genome [10,22]. In eukaryotes, tandem repeated sequences, such as the microsattellites of 1-15 bp and minisatellites of 10-100 bp, have been found and used clinically [20]. In mycobacteria, repeated sequences similar to the minisatellites in eukaryotes have been identified from the genome sequences of M. tuberculosis H37Rv and M. bovis AF2122/976 [4]. Based on the minisatellites of mycobacteria, the VNTR methods have been applied to typing M. bovis. One VNTR method is the mycobacterial interspersed repetitive units (MIRUs). MIRUs are scattered across 41 locations in the M. tuberculosis H37Rv genome and these are composed of 51-77 bp repetitive sequences [20]. Twelve of the 41 loci have shown polymorphism and these 12 loci have been used for typing the M. tuberculosis complex [11,20]. Additionally, Queen's University Belfast (QUB) VNTRs have been applied to M. bovis strains [15,19]. The VNTR typing methods have shown good stability, reproducibility and high discriminatory power for the M. tuberculosis complex [10,12].
In this study, we applied the VNTR typing methods on M. bovis strains that were isolated in Gyeonggi-do, Korea and we examined the prevalence and distribution of the genotypes of the M. bovis isolates.
Fifty-nine M. bovis isolates originating from dairy cattle that had tuberculosis-like lesions, and the cattle were from Gyeonggi-do livestock, were included in this study; the isolates were taken by the Veterinary Service from 2003 to 2004. Samples from the hilar lymph nodes of cattle suspected to be infected with bovine TB were collected, homogenized with sterile saline solution and decontaminated with N-acetyl-L-cysteine-4% NaOH for 15 min at room temperature. After centrifugation at 1,000 × g for 20 min, the isolates were cultured on Lowenstein-Jensen media (Difco, USA) for 3 to 4 weeks at 37℃. Bacteriological identification of M. bovis was based on acid-fast staining, the nitrate reductase test and the tiophene-2-carboxylic acid hydrazine assay (Becton Dickinson, USA). M. tuberculosis H37Rv (ATCC 27294) was used as a reference strain because its genomic sequence information is available.
The genomic DNA was extracted from the M. bovis isolates as described [15]. In brief, the M. bovis isolates from the slopes of the Lowenstein-Jensen media were grown for 3 to 4 weeks at 37℃ in Middlebrook 7H9 liquid medium (Difco, USA) that was supplemented with oleic acid-albumin-dextrose-catalase and Tween 80. The cultures of M. bovis were collected by centrifugation at 10,000 × g for 10 min and they were resuspended with 250 µl of sterile distilled water. The suspended cultures were boiled for 5 min in a water bath, and the supernatant was collected after removing the cellular debris by centrifugation. The DNA concentration was measured with a spectrophotometer at wavelength of 260 nm (Pharmacia Biotech., USA). The DNA was kept at -20℃ until it was used for PCR reaction.
Smart Taq Pre-Mix (Solgent, Korea) was used for the PCR amplification. Twelve MIRU, 3 ETR (A to C), 7 QUB (11a, 11b, 18, 26, 1895, 3232, 3336), 2 VNTR (0424, 1955) primer pairs were used (Table 1). The PCR reaction was performed with a 20 µl PCR mixture that contained 10 mM Tris-HCl, 50 mM KCl, 1.5 mM MgCl2, 200 µM of each dNTP, 0.5 µM of each primer, 1.5 units of Taq DNA polymerase (Perkin-Elmer Biosystems, USA) and 20 ng genomic DNA as a template. PCR amplification was carried out in a Geneamp PCR system 2700 (Applied Biosystems, USA). The initial denaturation at 95℃ for 10 min was followed by 30 cycles of 30 sec at 94℃, 60 sec at 58℃ and 90 sec at 72℃. The reaction was terminated by a 7 min step at 72℃. Genomic DNA of M. tuberculosis H37Rv and sterile distilled water were used as the positive and negative controls, respectively, in each set of reactions. The PCR products were analyzed by performing electrophoresis with using 1.5% agarose gels in 1 × Tris-boric acid-EDTA buffer (pH 7.2). The TriDye 100-bp DNA ladder (New England Biolabs, USA) was used for estimating the size of the PCR products.
The discriminatory power of the individual and combined VNTR markers was assessed by calculating the allelic diversity (h) with using the following equation: h = 1 - Σ χi2[n/(n-1)], where n is the number of isolates and xi is the frequency of the ith allele at the locus [17].
All 59 M. bovis strains were isolated from 20 dairy cattle farms in Gyeonggi-do, Korea. MIRU-VNTR analysis was performed on all the 59 M. bovis isolates by using 24 published markers (Table 1), which included 12 MIRU, 3 ETR, 7 QUB and 2 VNTR loci. Ten of the 24 VNTR markers showed genetic polymorphism (Table 2). The loci that showed polymorphism were two MIRU (26, 31), two ETR (A, B), five QUB (18, 26, 1895, 3232, 3336), and one VNTR (0424). Twelve different VNTR profiles were obtained by these 10 loci.
The allelic diversity (h) differed for the individual loci, ranging from 0.00 to 0.64 (Table 3). The QUB 3336 locus showed the highest discriminatory power (h = 0.64, Fig. 1). QUB 26 and MIRU 31 also showed high allelic diversity (h = 0.35), and four other loci (MIRU 26, ETR B, QUB 1895, VNTR 0424) showed low discriminative power (h = 0.02-0.05). In 12 MIRUs, two loci displayed allelic diversity and the other ten loci showed no allelic diversity. In the QUBs, 5 of 7 loci showed allelic diversity. The ETRs and QUBs had more polymorphic regions than did the MIRUs in the M. bovis isolates from Gyeonggi-do, Korea.
The discriminatory power of combining the VNTR loci was evaluated and compared (Table 4). When all the VNTRs were combined together, there were 12 different alleles and the allelic diversity (h) was 0.86. The combination of the three most polymorphic VNTR markers (MIRU 31, QUB 26, QUB 3336) showed three different alleles and high allelic diversity (h = 0.64). When the discriminative loci (ETR A, QUB 18 and QUB 3232) were added to this combination (MIRU 31, QUB 26, QUB 3336), nine different alleles were identified and the allelic diversity was enhanced (h = 0.84). With using all the QUBs and all the ETRs, eight and five different alleles were identified and the allelic diversities (h) were 0.77 and 0.57, respectively. With using all 12 MIRUs, three different alleles were identified and the allelic diversity (h) was 0.38.
The VNTR profiles of the 59 M. bovis isolates were examined. Twelve genotypes were identified from the M. bovis isolates originating from the 20 dairy cattle farms in Gyeonggi-do.
Most of the farms had one genotype that was identical to the M. bovis isolates in the region of Gyeonggi-do (Fig. 1). Interestingly, there were three farms that had two M. bovis genotypes (Table 5). Two genotypes (designation index (d.i.) = c, e) coexisted at farm C. There were multiple TB outbreaks at farms D, F and H. The genotype (d.i. = g) of the M. bovis isolates was identical at the first and second outbreaks on farm H. Interestingly, the genotype of M. bovis was different between the first (d.i. = b) and second outbreaks (d.i. = d) on farm D. The identical genotype (d.i. = c) of the M. bovis isolates was identified at the first and second outbreaks, but a new genotype (d.i. = f) of M. bovis appeared at the third outbreak as the main genotype with a previous genotype (d.i. = c) being noted at farm F.
The prevalence of the genotypes of the M. bovis isolates was examined. Two genotypes of the M. bovis isolates (d.i. = e, g) were prevalent in Gyeonggi-do. These two genotypes of the M. bovis isolates were identified at four cattle farms (20% of the total cattle farms) and they accounted for 20.34% and 18.64% of the total M. bovis isolates, respectively. Two genotypes (d.i. = c, f) of the M. bovis isolates accounted for about 15% of the total M. bovis isolates individually and these were found at 2-3 cattle farms. Most of the remaining genotypes were identified from one M. bovis isolate at one cattle farm (Fig. 2, Table 6).
Advances in molecular typing techniques have contributed to the epidemiological study of infectious diseases such as bovine TB. Molecular typing techniques have enabled us to identify and distinguish M. bovis isolates, and the data from molecular typing allow us to discern the genotypes of M. bovis that are prevalent in a specific area and how multiple outbreaks occur. Moreover, this epidemiological information could trace the origin of outbreaks and advise us on how to block the transmission of bovine TB. The MIRU-VNTR typing is a powerful tool for identifying and genotyping the M. tuberculosis complex. Various combinations of tandem repeats such as MIRUs, ETRs and VNTRs have been proven to be useful for identifying the M. tuberculosis complex, including M. bovis [7,16,21]. Spoligotyping is the method to detect the presence or absence of a 43 spacer DNA sequence between direct repeats (DR) in the DR region of M. tuberculosis complex strains, and IS6110 typing is a RFLP method that uses the element IS6110 as a probe. Spoligotyping and IS6110 RFLP are also useful tools, but spoligotyping and IS6110 RFLP have been reported to be less discriminative that the other typing methods for M. bovis because most of the M. bovis isolates have one or few copies of DR or IS6110, respectively [15,18]. The MIRU-VNTR method has proven to be more reproducible, stable and sensitive for the M. tuberculosis complex than IS6110 RFLP [9].
In this study, we analyzed 59 M. bovis isolates from Gyenggi-do, Korea by using 24 published novel VNTR markers. Among the four sets of the MIRU-VNTR loci, the ETRs and QUBs have shown high polymorphism, but the MIRUs have shown very low polymorphism. This result is consistent with previous studies that the QUBs and ETRs showed highly discriminative power both for M. bovis and M. tuberculosis [5,19], yet the MIRUs have a high discriminative power for M. tuberculosis, but not for the M. bovis isolates [1,3,7,11,21]. Three loci (QUB 26, QUB 3336, MIRU 31) showed high discriminative power (h = 0.35, 0.64, 0.35, respectively), and ETR A, QUB 18 and QUB 3232 showed moderate allelic diversity (0.26, 0.25, 0.18, respectively). This result is consistent with other reports that five loci (QUB 3232, ETR (A, B), MIRU (24, 26)) were highly discriminative for the M. bovis isolates from Chad [7], and six loci, QUB (11a, 26, 3232), ETR (A, B), MIRU 26, were highly polymorphic for the M. bovis isolates from Northern Ireland [16]. The M. bovis isolates from Belgium were highly discriminated by seven loci, QUB (11a, 11b, 3232, 3336), ETR (A, B), MIRU 26 [1]. However, QUB 11a and MIRU 26 showed low discriminative power for the M. bovis isolates from Korea in our study. Also, MIRU 31 (corresponding to ETR E) showed higher polymorphism (h = 0.35) for the M. bovis isolates from Korea than that reported in previous study [7].
There were 12 genotypes of M. bovis, and these genotypes were dispersed throughout the region of Gyeonggi-do, Korea. There were two prevalent genotypes (d.i. = e, g) of M. bovis in the area of Gyeonggi-do, and these two main genotypes were identified in about 20% of the 59 total M. bovis isolates and at 20% of the total 20 total cattle farms. This data could not be compared because there have been no previous studies on the genotypes of M. bovis isolates in Korea.
There were three cattle farms on which multiple outbreaks took place. One outbreak took place because of a relapse of a previous M. bovis strain, and another outbreak took place successively because of two different genotypes of the M. bovis strains. Interestingly, the pattern of the outbreaks at one cattle farm indicated a combination of the relapse of a previous M. bovis strain and the introduction of a new genotype of the M. bovis strain. Two M. bovis genotypes coexisted at the third outbreak on this farm, and this might reflect the current state of the Korean livestock industry.
This study suggests the VNTR markers that have high discriminative power could be used to investigate the M. bovis isolates from all over Korea, and the information on the genotypes of the M. bovis isolates might provide important information on how to control bovine TB in Korea.
Figures and Tables
Fig. 1
The geographical origin of the M. bovis strains isolated from cattle are aligned with the corresponding VNTR genotype. A capital letter indicates the cattle farm. A lower-case letter indicates the genotypes of the M. bovis strains at each cattle farm and the number in parenthesis indicates the number of outbreaks of the genotype of the M. bovis isolates.
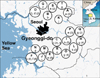
Fig. 2
PCR products of the various M. bovis isolates with using primers that amplify QUB3336. Lane M: 100 bp DNA ladder, lane 1-12 and lanes 15-26: M. bovis isolates, lanes 13 and 27: M. tuberculosis H37Rv, lanes 14 and 28: negative controls.

Acknowledgments
This work was supported in part by a grant from the Korean Health 21 R&D Project, the Ministry of Health and Welfare, Korea (A010381), and by a grant from the Brain Korea Project for Medical Sciences at Yonsei University.
References
1. Allix C, Walravens K, Saegerman C, Godfroid J, Supply P, Fauville-Dufaux M. Evaluation of the epidemiological relevance of variable-number tandem-repeat genotyping of Mycobacterium tuberculosis and comparison of the method with IS6110 restriction fragment length polymorphism analysis and spoligotyping. J Clin Microbiol. 2006. 44:1951–1962.

2. Alito A, Morcillo N, Scipioni S, Dolmann A, Romano MI, Cataldi A, van Soolingen D. The IS6110 restriction fragment length polymorphism in particular multidrug-resistant Mycobacterium tuberculosis strains may evolve too fast for reliable use in outbreak investigation. J Clin Microbiol. 1999. 37:788–791.

3. Cowan LS, Mosher L, Diem L, Massey JP, Crawford JT. Variable-number tandem repeat typing of Mycobacterium tuberculosis isolates with low copy numbers of IS6110 by using mycobacterial interspersed repetitive units. J Clin Microbiol. 2002. 40:1592–1602.

4. Domenech P, Barry CE III, Cole ST. Mycobacterium tuberculosis in the post-genomic age. Curr Opin Microbiol. 2001. 4:28–34.
5. Frothingham R, Meeker-O'Connell WA. Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem DNA repeats. Microbiology. 1998. 144:1189–1196.

6. Heersma HF, Kremer K, van Embden JD. Computer analysis of IS6110 RFLP patterns of Mycobacterium tuberculosis. Methods Mol Biol. 1998. 101:395–422.
7. Hilty M, Diguimbaye C, Schelling E, Baggi F, Tanner M, Zinsstag J. Evaluation of the discriminatory power of variable number tandem repeat (VNTR) typing of Mycobacterium tuberculosis strains. Vet Microbiol. 2005. 109:217–222.

8. Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden JDA. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997. 35:907–914.

9. Kremer K, van Soolingen D, Frothingham R, Haas WH, Hermans PWM, Martín C, Palittapongarnpim P, Plikaytis BB, Riley LW, Yakrus MA, Musser JM, van Embden JD. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis complex strains: interlaboratory study of discriminatory power and reproducibility. J Clin Microbiol. 1999. 37:2607–2618.

10. Le Flèche P, Fabre M, Denoeud F, Koeck JL, Vergnaud G. High resolution, on-line identification of strains from the Mycobacterium tuberculosis complex based on tandem repeat typing. BMC Microbiol. 2002. 2:37.
11. Mazars E, Lesjean S, Banuls AL, Gilbert M, Vincent V, Gicquel B, Tibayrenc M, Locht C, Supply P. High-resolution minisatellite-based typing as a portable approach to global analysis of Mycobacterium tuberculosis molecular epidemiology. Proc Natl Acad Sci USA. 2001. 98:1901–1906.

12. O'Reilly LM, Daborn CJ. The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tuber Lung Dis. 1995. 76:Suppl 1. 1–46.
13. Perumaalla VS, Adams LG, Payeur J, Baca D, Ficht TA. Molecular fingerprinting confirms extensive cow-to-cow intra-herd transmission of a single Mycobacterium bovis strain. Vet Microbiol. 1999. 70:269–276.

14. Roring S, Brittain D, Bunschoten AE, Hughes MS, Skuce RA, van Embden JDA, Neill SD. Spacer oligotyping of Mycobacterium bovis isolates compared to typing by restriction fragment length polymorphism using PGRS, DR and IS6110 probes. Vet Microbiol. 1998. 61:111–120.

15. Roring S, Scott A, Brittain D, Walker I, Hewinson G, Neill S, Skuce R. Development of variable-number tandem repeat typing of Mycobacterium bovis: comparison of results with those obtained by using existing exact tandem repeats and spoligotyping. J Clin Microbiol. 2002. 40:2126–2133.

16. Roring S, Scott AN, Glyn HR, Neill SD, Skuce RA. Evaluation of variable number tandem repeat (VNTR) loci in molecular typing of Mycobacterium bovis isolates from Ireland. Vet Microbiol. 2004. 101:65–73.

17. Selander RK, Caugant DA, Ochman H, Musser JM, Gilmour MN, Whittam TS. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986. 51:873–884.

18. Serraino A, Marchetti G, Sanguinetti V, Rossi MC, Zanoni RG, Catozzi L, Bandera A, Dini W, Mignone W, Franzetti F, Gori A. Monitoring of transmission of tuberculosis between wild boars and cattle: genotypical analysis of strains by molecular epidemiology techniques. J Clin Microbiol. 1999. 37:2766–2771.

19. Skuce RA, McCorry TP, McCarroll JF, Roring SMM, Scott AN, Brittain D, Hughes SL, Hewinson RG, Neill SD. Discrimination of Mycobacterium tuberculosis complex bacteria using novel VNTR-PCR targets. Microbiology. 2002. 148:519–528.

20. Supply P, Mazars E, Lesjean S, Vincent V, Gicquel B, Locht C. Variable human minisatellite-like regions in the Mycobacterium tuberculosis genome. Mol Microbiol. 2000. 36:762–771.

21. Supply P, Lesjean S, Savine E, Kremer K, van Soolingen D, Locht C. Automated high-throughput genotyping for study of global epidemiology of Mycobacterium tuberculosis based on mycobacterial interspersed repetitive units. J Clin Microbiol. 2001. 39:3563–3571.

22. van Deutekom H, Supply P, de Haas PE, Willery E, Hoijng SP, Locht C, Coutinho RA, van Soolingen D. Molecular typing of Mycobacterium tuberculosis by mycobacterial interspersed repetitive unit-variable-number tandem repeat analysis, a more accurate method for identifying epidemiological links between patients with tuberculosis. J Clin Microbiol. 2005. 43:4473–4479.
23. van Soolingen D, Hermans PWM, de Haas PEW, Soll DR, van Embden JDA. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol. 1991. 29:2578–2586.

24. van Soolingen D, Borgdorff MW, de Haas PE, Sebek MM, Veen J, Dessens M, Kremer K, van Embden JDA. Molecular epidemiology of tuberculosis in the Netherlands: a nationwide study from 1993 through 1997. J Infect Dis. 1999. 180:726–736.

25. van Soolingen D. Molecular epidemiology of tuberculosis and other mycobacterial infections: main methodologies and achievements. J Intern Med. 2001. 249:1–26.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download