Abstract
A 4-month-old female Holstein Friesian calf was referred to the Veterinary Teaching Hospital, University of Berne, Switzerland for evaluation of ataxia, weakness, apathy and stunted growth. Clinical examination revealed generalized ataxia, propioceptive deficits, decreased menace response and sensibility. Postmortem examination did not reveal macroscopic changes of major organs. Histologically, the brain and the spinal cord lesions were characterized by polymicrocavitation, preferentially affecting the white matter fibers at the junction of grey and white matter and by the presence of Alzheimer type II cells. The liver revealed lesions consistent with a congenital portosystemic shunt, characterized by increased numbers of arteriolar profiles and hypoplasia to absence of portal veins. The pathological investigations along with the animal history and clinical signs indicated a hepatic encephalomyelopathy due to a congenital portosystemic shunt.
Hepatic encephalopathy (HE) refers to a spectrum of neuropsychiatric abnormalities associated with significant liver dysfunction and is commonly described in humans and domestic carnivores [5,6]. In veterinary medicine, congenital portosystemic shunts (CPSSs) (Fig. 1) are the most common cause of HE in young dogs and cats [7,11], while in large animals HE is often described with various hepatotoxic conditions [14]. In large domestic animals, CPSSs have only been sporadically reported [2,4,9,10,13]. Generally, the clinical signs associated with CPSS in large animals are unspecific and mimic those of HE; including progressive depression, ataxia, lethargy, apparent blindness, slow proprioception, hindquarters weakness and abnormal behavior. There are many postulated mechanisms to explain the pathogenesis of HE, but there is a general consensus that ammonia plays an important role in the dysfunction of astroglial cells leading to brain edema [3,5,8]. This report describes the clinical and neurological investigations, and pathological findings in a Holstein Friesian calf with hepatic encephalo-myelopathy due to a CPSS.
A 2-month-old female Holstein Friesian calf was admitted to the Veterinary Teaching Hospital, University of Berne, Switzerland for evaluation of ataxia, weakness and stunted growth. On physical examination the animal was underweight with generalized muscle atrophy. Neurological examination showed generalized ataxia, propioceptive deficits, decreased menace response and superficial sensibility. Temperature, heart and breathing rates were at normal range. Cerebrospinal fluid analysis, blood and serum biochemical analyses including liver enzymes were also within normal limits. Electromyographic (EMG) examination revealed isolated spontaneous activity such as fibrillation potentials in the proximal musculature of front and hind limbs, which indicated a nonspecific denervating neuropathic or myopathic disease. The calf was treated during hospitalization with penicillin (40,000 IU/kg iv), dexamethasone (0.03 mg/kg iv; Boehringer Ingelheim, Germany) and thiamine (4.5 mg/kg; Vétoquinol, France) for 7 days. The animal recovered and was therefore taken home. Two months later it was returned to the Veterinary Hospital with a rapidly progressing debilitation. The animal was in left lateral recumbency, and dyspneic. Although the rectal temperature was normal (38.2℃), the heart and respiratory rates were relatively high at 128/beats per min and 80/breaths per min, respectively (normal ranges are 80 to 100 beats per min and 24 to 36 breaths per min) [12]. The general condition of the calf deteriorated rapidly with progression to stupor and coma. After poor prognosis was established, the calf was humanely euthanized.
Subsequently, a complete necropsy was performed, the analysis of which identified a poor staturoponderal development considering the age and the breed of the animal (measured weight 82 kg, normal weight 136 kg). The general body condition was reduced with generalized muscle atrophy. No other macroscopical lesion was observed. Representative tissue samples were fixed in 10% neutral buffered formalin, processed by routine methods in ascending grades of alcohol and embedded in paraffin wax. Sections were cut (4 µm) and stained with hematoxylin and eosin. Selected brain and spinal cord tissue samples were additionally stained with Luxol fast blue. Immunohisto chemistry was performed on brain and spinal cord sections with a streptavidin-biotin method using an anti-bovine glial fibrillary acid protein (GFAP) antibody (1:1,000 DAKO, Denmark). Paraffin wax-embedded brain and spinal cord tissues of one normal age-matched calf was used as control.
Histological examination of the CNS revealed an intense and widespread bilateral-symmetric spongy degeneration, primarily affecting the white matter with involvement of transitional areas between grey and white matter (Fig. 2). The lesion was diffusely distributed throughout the cerebrum, midbrain, cerebellum, brainstem and spinal cord. Spinal cord lesions were seen along the whole spinal cord involving primarily the myelinated fibers in the grey matter (Fig. 3). In the cerebral cortex and in the adjacent white matter, Alzheimer type II cells were observed as isolated clusters. Luxol fast blue staining of spinal cord tissue did not reveal any changes in myelin compound. Immunohistochemically, GFAP staining did not show any obvious differences in staining intensity in comparison with a normal control calf.
Microscopically, the hepatic portal triads presented numerous prominent proliferating small arterioles, hypoplastic to absent portal veins, proliferation of bile ducts and often ectasia of lymphatic vessels (Fig. 4). In cross-sections, skeletal muscles revealed diffusely atrophic myofibers (up to 30 µm in diameter) with occasionally single group of angular and shrunken atrophic myofibers. Peripheral nerves were histologically unremarkable.
The pathomorphological lesions described herein are compatible with a hepatic encephalomyelopathy due to a CPSS with secondary generalized muscle atrophy. HE is described in association with hepatic failure and consists of bilateral symmetric spongy degeneration of white matter and the presence of single or small groups of Alzheimer type II cells [11]. Hepatic myelopathy is rarely described in veterinary medicine. A similar myelopathy as decribed in this case has only been described in experimentally induced hyperammonemic calves [1]. In human medicine, hepatic myelopathy is defined as a neurological complication of hepatic cirrhosis [5] with portal hypertension, usually characterized by symmetrical demyelination of the corticospinal tracts [5]. In this case, the most pronounced spinal cord changes were restricted to the nerve fibers in the spinal cord grey matter without obvious demyelination which differs significantly to that found in human patients.
Hepatic atrophy and intra- or extra-hepatic vascular anomalies are the main gross findings reported in large animal species with CPSS [4,13]. Ascites, due to persistent portal hypertension, is commonly associated with acquired portosystemic shunts, and is absent in congenital portosystemic shunts [14]. The histological findings of the liver together with the absence of ascites, multiple tortuous shunted vessels or hepatic changes were compatible with a CPSS, similarly to previous reports [9].
Due to the absence of clinicopathological evidence of hepatic dysfunction in this calf and to the unspecific clinical signs, a portosystemic shunt with secondary hepatic encephalopathy was not included in the differential diagnosis. However, total serum bile acid concentration, total blood ammonia values and sulfobromophthalein retention time tests, normally increased in CPSS, were not measured. The clinical signs observed in this reported calf (ataxia, depression, stupor and coma) could be explained by the hepatic encephalomyelopathy. However, clinical signs were far unspecific which led to a erroneous diagnosis. The muscle atrophy in this calf was most likely related to the CPSS, as severe liver disease are known to induce protein metabolic perturbations and secondary muscle atrophy [14]. Although EMG results suggested a peripheral nervous system involvement, peripheral nerves did not reveal any histopathological changes. However, the presence of single group of atrophic myofibers in histology suggested a mild neurogenic atrophy, probably correlated to the severe spongy changes in adjacent areas of the motor neurons in the grey matter. The possible explanation for the initial recovery after antibiotic administration may be that ureaseproducing intestinal bacteria were temporarily reduced in the intestinal tract, hence long-term medical management using oral antibiotics has been described in small animals to inhibit both the production and absorption of potential gut derived CNS toxins [7].
Congenital portosystemic shunt with secondary hepatic encephalomyelopathy has rarely been described in calves. However, hepatic encephalomyelopathy due to CPSS might have heterogeneous manifestations. Because of the nonspecific associated clinical signs, it is likely that CPSS is often under-diagnosed. Hence, it should be considered in the differential diagnostic list in young animals exhibiting stunted growth and history of intermittent neurological deficits.
Figures and Tables
Fig. 1
Simplified drawings illustrating types of portosystemic shunts. (A) Normal liver. (B) Intrahepatic portosytemic shunt. (C) Extrahepatic portosystemic shunt.
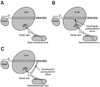
Fig. 2
Photomicrographs of calf cerebrum. (A) Capsule interna. Note diffuse severe spongy vacuolation in the adjacent areas between grey and white matter. H&E stain. Scale bar = 1 mm. (B) The capsule interna showing severe white matter changes. gm; grey matter. wm; white matter. H&E stain. Scale bar = 100 µm.
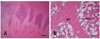
Fig. 3
Photomicrographs of calf spinal cord. (A) Bilateral symmetric diffuse spongy vacuolation of the grey matter. White matter funiculi not affected. H&E stain. Scale bar = 500 µm. (B) The grey matter intermediate horn showing spongy changes adjacent to neuron. H&E stain. Scale bar = 50 µm.
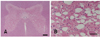
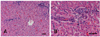
Fig. 4
Photomicrographs of calf liver. (A) Note a hepatic lobule surrounded by numerous portal spaces with absence of portal veins. The distance between portal spaces and central vein is abnormally reduced. H&E stain. Scale bar = 50 µm. (B) The portal area showing proliferation of arterioles and ductules with absence of portal vein. H&E stain. Scale bar = 50 µm.
Acknowledgments
The authors thank Dr. A. Zakher for reviewing the manuscript and Mr. Joerg Jenni for technical support.
References
1. Cho DY, Leipold HW. Experimental spongy degeneration in calves. Acta Neuropathol. 1977. 39:115–127.

2. Fortier LA, Fubini SL, Flanders JA, Divers TJ. The diagnosis and surgical correction of congenital portosystemic vascular anomalies in two calves and two foals. Vet Surg. 1996. 25:154–160.

3. Hooper PT. Spongy degeneration in the central nervous system of domestic animals. Part I: Morphology. Acta Neuropathol. 1975. 31:325–334.

4. Keane D, Blackwell T. Hepatic encephalopathy associated with patent ductus venosus in a calf. J Am Vet Med Assoc. 1983. 182:1393–1394.
7. Martin RA. Congenital portosystemic shunts in the dog and cat. Vet Clin North Am Small Anim Pract. 1993. 23:609–623.

8. Norenberg MD. Astroglial dysfunction in hepatic encephalopathy. Metab Brain Dis. 1998. 13:319–335.
9. Olchowy TW, Daniel GB, Tucker RL, Petersen MG. Hepatic vascular anomaly in a calf. Can Vet J. 1992. 33:131–133.
10. Reimer JM, Donawick WJ, Reef VB, Wagner HR, Divers TJ. Diagnosis and surgical correction of patent ductus venosus in a calf. J Am Vet Med Assoc. 1988. 193:1539–1541.
11. Summers BA, Cummings JF, de Lahunta A. Summers BA, Cummings JF, de Lahunta A, editors. Degenerative diseases of the central nervous system. Veterinary Neuropathology. 1994. St. Louis: Mosby;189–350.
12. Terra RL. Smith BP, editor. Ruminant history, physical examination and medical records. Large Animal Internal Medicine: Disease of Horses, Cattle, Sheep and Goats. 1996. 2nd ed. St. Louis: Mosby;10–11.
13. Van den Ingh TS, van der Linde-Sipman JS, Berrocal A, Vos JH. Congenital portosystemic shunts in three pigs and one calf. Vet Pathol. 1990. 27:56–58.

14. Zachary JF. McGavin MD, Zachary JF, editors. Nervous system. Pathologic Basis of Veterinary Disease. 2007. 4th ed. St. Louis: Mosby-Elsevier;883–971.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download