Abstract
The production of miniature animals has been suggested for use in organ transplantation. At present, many of the studies about application of animal organs to human have been focused on pigs because of the number of advantages involved and due to their similarities with human. However, a physiological analysis of the organs to be transplanted has not yet been carried out. Therefore, this study analyzed whether or not there were physiological and morphological differences in the hearts of conventionally-reared pigs and micropigs. In this study, the morphological and physiological functions of the heart were examined using radiographic and echocardiographic equipment. In the lateral radiographic view, the heart of the micropig has a larger cardiac long axis : short axis ratio than does the conventional pig, but the difference in the vertebral heart score was not significant. In addition, there were no morphological differences on the X-ray fluoroscopic view. There were no differences in echocardiographic values, except for several values in the left ventricle traces. Overall, it is expected that the values measured in this study will contribute to understanding of the physiological characteristics of micropigs.
The severe shortage of human donor organs has stimulated interest in the potential use of animal organs for transplantation into humans [6]. Xenotransplantation, as such, would not only offer an unlimited supply of organs and tissues for transplantation, but might also provide an opportunity to carry out transplantation without the problems associated with infections or disease conditions, which would inevitably occur in human transplants. On the other hand, one essential question in xenotransplantation is whether, and to what extent, an animal organ or tissue can provide a physiologic replacement for a human organ or tissue. Foreign organs and tissues might function poorly compared with their human counterparts. However, it is equally intuitive that the optimal functioning of an organ transplant is not often achieved and is rarely necessary.
The evolution of mammals has resulted in increasing functional specialization. Most of the physiological incompatibilities and species-specific differences between mammal species are unknown. Targeted investigations suggest that even individuals of one species or strain may demonstrate slight genetically-based metabolic differences. This is particularly true for widely divergent species, which show significant and multiple incompatibilities due to their evolutionary development. This is not restricted to differences in molecules, hormones, or enzymes and their receptors, but also to species-specific products that will prove antigenic. Therefore, the question as to whether xenogeneic donor organs will be physiologically compatible with human hosts needs to be addressed, since even the suboptimal functioning of one or several systems may cause life-threatening problems to the xenografted patient. There are considerable similarities between the porcine and human anatomy and physiology. The cardiac outputs of porcine and human hearts of similar size have been found to be similar [8], and their action potentials are also similar [17]. However, a recent comparison of the anatomy of porcine and human hearts has identified several differences, many of which are due to the different stances (walking on four legs rather than two; unguligrade versus orthograde) and the effect of gravity on the development of the heart. Despite these differences, the transplanted organs appear to adapt very well to new hosts, for example pig hearts in monkey hosts. However, there is limited data on the functioning of the porcine heart in primates. Recently, Bhatti et al. [2] reported that porcine hearts could provide physiological support in non-human primates for up to three months.
The echocardiographic examination has been the cornerstone of cardiac diagnosis in a variety of cardiovascular disorders for over two decades. Two-dimensional and Doppler echocardiographic modalities provide valuable information on the cardiac structures, chamber function, and hemodynamic derangements. This report is the first to show that echocardiography is a much more sensitive method for assessing micropig cardiac functions for xenografts in nonhuman primates than palpation.
All animal experiments were carried out in accordance with the Standard Operation Procedures of the Institutional Animal Care and Use Committe, Seoul National University, Korea. The studies were performed with mixed-breed, conditioned Yucatan micropigs and Landrace breed conventional pigs, which were purchased from PWG Genetics (Korea). Prior to their purchase, the pigs were physically examined and confirmed to be healthy. The pigs were individually housed indoors in cages, fed dry pig food, and provided with water ad libitum. The mean age of the experimental animals was 80 days for the conventional pigs and 360 days for the micropigs. The mean body weight was 30.50 ± 0.45 kg in the conventional pigs and 32.10 ± 1.29 kg in the micropigs (Table 1). Blood pressure was measured five times for each individual animal with Cardell BP monitor (Sharn Veterinary, USA) in the premedicated condition with atropine (0.04 mg/kg IM), xylazine (2.2 mg/kg IM), and a zolazepam/tiletamine cocktail (4.4 mg/kg IM) prior to echocardiographic examination.
The animals were fasted overnight and premedicated with atropine, xylazine, and a zolazepam/tiletamine cocktail, intubated, mechanically ventilated, and maintained with isoflurane (1% to 5%) inhalational general anesthesia. There is a good correlation between the body length and heart size regardless of the chest conformation. Because of this relationship, the vertebral heart score (VHS) can be used to both determine the presence and quantify the degree of cardiomegaly in animals. The VHS measurements were carried out using the lateral chest radiograph.
The VHS was evaluated by transposing the long-axis (L) and short-axis (S) heart dimensions onto the vertebral column, and recording the number of vertebra, beginning with the cranial edge of the fourth thoracic vertebra. The VHS was calculated using the following formula:
VHS = L + S [3].
X-ray fluoroscopy was carried out to observe the cardiac blood flow of the right circumflex artery, left anterior descending artery, and left circumflex artery. Cardiac catheterization was performed on anesthetized animals. An 8Fr arterial sheath was then inserted through the carotid artery after local anesthesia with 2% lidocaine, and a cutdown was made. The 7 or 8Fr coronary guiding catheter and guide-wire assembly in the coronary ostium was engaged under fluoroscopic guidance using a Phillips C-arm system (BV-25 Gold). The long guide wire was used to introduce a pigtail infusion catheter, and the pigtail catheter was positioned in the left ventricle to infuse iohexol as a contrast medium. X-ray fluoroscopy was then performed.
Echocardiographic examinations were carried out using a cardiac ultrasound system equipped with a 5.0-7.5 MHz transducer (GE LogiQ 7; GE Medical Systems, USA). The echocardiographic images were obtained in the right parasternal long- and short-axis view. The anatomy and function were measured in various cardiac regions including four cardiac chambers, valves, and great vessels by B-mode, M-mode, and spectral Doppler echocardiographic tracing. The dimensions and thicknesses of the cardiac structures were measured using M-mode echocardiography according to the American Society of Echocardiography guidelines. The blood flow velocity, amounts of blood flow, pressure gradients across the valves, and stroke volume were measured using pulsed or continuous wave Doppler echocardiography. The cardiac output was calculated by multiplying the stroke volume by the heart rate [5].
The left ventricular (LV) systolic function was evaluated by measuring the ejection fraction (EF) and fractional shortening (%FS) by M-mode echocardiography according to the following formulas:
EF = (End-diastolic volume - end-systolic volume)/end-diastolic volume × 100,
%FS = (LV end-diastolic dimension - LV end-systolic dimension)/LV end-diastolic dimension × 100 [7].
All measured values used in the final calculations for each pig are the mean value from three to five sequential measurements regarded as being of the optimal quality.
Echocardiography was performed on the micropigs while they were under general anesthesia and mechanical ventilation. As compared to the human or small laboratory animals (rat, mouse), the pig heart long axis is "rotated" posteriorly in the thorax; hence, the right ventricle appears posterior to the left ventricle. The parasternal short- and long-axis views were readily obtained. While imaging from the long-axis view, the atrioventicular valves and the left ventricle outflow tract were imaged to obtain flow measurements through the aortic valve.
Table 1 shows the clinical and laboratory characteristics of the study population. There were no significant differences between the vital signs of the conventional pigs and micropigs. The mean systolic arterial pressure was 122.15 ± 3.50 mmHg in the conventional pigs and 121.79 ± 3.64 mmHg in the micropigs under a premedicated condition. The morphological differences between the conventional pig and micropig were compared using radiological examinations. As shown Fig. 1, thoracic radiography was carried out to measure the VHS. The VHS can be used to determine the range of normal cardiac dimensions in micropigs. No significant difference was observed in the length of the long axis, but there was a difference in the length of the short axis. In the micropig, the cardiac length of the short axis was shorter than in the conventional pig, but a significant difference in the VHS was not observed (Table 2). X-ray fluoroscopy was also performed to assess the blood flow and morphologic characteristics of the cardiac blood vessels. However, no significant differences were found between the two groups (Fig. 3). In the series of echo sections, each segment of the heart was visualized using transthoracic echocardiography. Fig. 4 shows the measured and calculated parameters from two-dimensional echocardiography. The apical 5-chamber view was easily obtained in all animals. The hearts of all animals were structurally normal. Tables 3, 4, 5 show the measured physiological cardiac functions. There were no significant differences observed between the conventional pigs and micropigs for nearly every measured parameter, except in the LV trace. The LV outflow track velocity, gradient, and LV fraction shortening were significantly lower in the micropigs than in the conventional pigs. In addition, we compared cardiac function of the micropig with that of the human. As shown Tables 6, 7, 8, there were similarities in the echocardiographic values between human and micropigs.
Accurately evaluating cardiac function in a clinical setting is a constant challenge. Highlights from numerous presentations on the use of echocardiography have led to the conclusion that this technique is becoming a commodity. From the considerable number of presentations, ubiquitous use is clearly the direction, not only in the noninvasive imaging section, but throughout the program, be it in novel percutaneous procedures such as valve implantation or in established procedures such as cardiac resynchronization therapy. However, this is far from the reality given the plethora of new advances being made in the field of echocardiography.
In this study, we performed echocardiography in pigs using an open chest model [10,16], and transesophageal and transthoracic echocardiography (personal experience). However, there are few reports of transthoracic echocardiography of a pig [18,20]. Transthoracic echocardiography of a micropig is technically challenging for several reasons. The close proximity of the ribs necessitates a small transducer footprint. The thorax is more oval-shaped in the anterior-posterior direction, and the long axis of the heart follows an anterior-posterior direction. These differences between humans and pigs are related to the basic differences in body orientation, with the pig having an unguligrade posture and the human having an upright posture [4]. Finally, the animal is usually mechanically ventilated under general anesthesia, which makes transthoracic echocardiographic imaging difficult. An evaluation of the right ventricle, through an assessment of the right ventricle free wall motion [18], and left ventricular function from a parasternal view [19] has been described. Parasternal short-axis views have been described as requiring the two-dimensional short-axis image to be "visually approximated by the closest fitting ellipse at the end-diastole and end-systole" [11]. Apical views have been reported as being impossible to obtain.
This study assessed the physiological characteristics of the heart of a micropig and compared the data with that of a conventional pig. The vertebral heart scores (VHSs) of each group were not significantly different, but the cardiac long axis : short axis ratio was larger in the micropigs than in the conventional pigs (Fig. 1, Table 2). This suggests that the cardiac shape of the micropigs was slightly different from that of conventional pigs. However, more investigations will be needed to be conducted to assess cardiac shape. The echocardiographic study showed that there were no the significant differences between the conventional pigs and micropigs, except for several values of the mitral valve and left ventricle. However, the general aspects of the echocardiographic values observed for the micropigs were lower than those of the conventional pigs. These aspects may not only be due to differences in cardiac shape, but also to the different sizes of the hearts. It was previously reported [13] that the weight of the heart in micropigs was lower than that in conventional pigs with an identical body weight. In addition, the fractional shortening results of micropigs in this study showed lower values than those measured in conventional pigs. The most common clinical methods for assessing the systolic ventricular function are the LV ejection phase indices [14]. None of these indices act as a specific measure of the LV contractility. Instead, they are measures of the global left ventricular performance. As such, they are as easily altered by changes in the preload and afterload as they are by the contractility [1]. Several indices of the LV function can be calculated from the LV dimensions measured from the M-mode or two-dimensional echocardiogram [9]. The LV fractional shortening is the most commonly used one-dimensional assessment, and is the simplest and most often used index of the LV systolic function. Moreover, the left ventricular velocities and gradients of the micropigs were lower than those of the conventional pigs. Therefore, the differences in the LV function, including those of fractional shortening, between two groups might be due to differences in the systolic function. The differences in these aspects may be result from the decreased development of the cardiac function due to the breeding environments of the micropigs, including limited movement.
Physiological differences may also pose problems. While the handling of sodium and potassium by pig and human kidneys is similar, there are differences in the normal serum calcium and phosphate concentrations. These simple metabolic incompatibilities may not be of great significance, but multicellular organisms also require cells to be able to communicate with each other through hormones and other molecules. Moreover, little is known about these more complex cross-species compatibilities. The question as to whether the pig heart is functionally and anatomically similar to its human counterpart was investigated [4]. In this study, we could observe that there are similarities in the echocardiographic values between humans and micropigs. The cardiac outputs of porcine and human hearts of similar size were found to be comparable, and their action potentials were also similar. However, the innervation and overall morphology of the atrioventricular node in pigs was significantly different from that in humans. This may create problems terms of the control of the heart rate and contractility in a human patient. Harmful arrhythmias within an extrinsically denervated donor pig heart may be greatly increased. The full spectrum of physiological incompatibilities is not completely understood. However, the differences could be amenable to other treatments, and may not be an insurmountable barrier.
In this study, two limitations should be noted. First, we used different age groups to compare the heart function of micropigs with another group having identical body weights. Several reports showed that there were not significant differences between different groups with identical body weights [7,15]. Second, we used the chemical restraining method to control the movement of pigs, and maintained animals general anesthesia using isoflurane (1% to 5%). Isoflurane is known to be an inhalable anesthetic drug that maintains the cardiovascular function well in pigs, and does not induce disarrythmia by epinephrine compared with other inhalable anesthetic drugs such as halothane or enflurane [12].
In conclusion, echocardiography imaging of the micropig is possible, and has potential utility for laboratory investigators. In particular, the parasternal images are readily obtainable for assessing the LV function, atria, and atrioventricular valves, as well as flow measurements through the left ventricle outflow.
Figures and Tables
Fig. 1
Lateral radiographic view of a conventional pig and a micropig. The images show the vertebral heart score (VHS) measurement method using the lateral chest radiograph. A: representative picture of conventional pig, B: representative picture of micropig. L: long-axis heart dimension, S: short-axis heart dimension, T4: fourth thoracic vertebra.
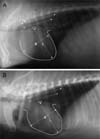
Fig. 2
Delivery of iohexol with X-ray fluoroscopic guidance. A: Right circumflex artery (RCA; white arrow) of a conventional pig, B: Left anterior descending artery (LAD; white arrow with dotted line) and left circumflex artery (LCX; black arrow) of a conventional pig, C: Right circumflex artery (RCA; white arrow) of a micropig, D: Left anterior descending artery (LAD; white arrow with dotted line) and left circumflex artery (LCX; black arrow) of a micropig.
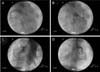
Fig. 3
Representative images of echocardiography. A: pulmonic valve trace, B: right ventricle trace, C: aortic valve trace, D, E: mitral valve trace, F, G: left ventricle trace.
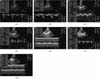
Table 3
Comparison of the values for the conventional pigs and micropigs measured by M-mode echocardiography

Table 4
Comparison of the values for conventional pigs and micropigs measured by 2D echocardiography

Table 5
Comparison of the values of conventional pigs and micropigs measured by Doppler echocardiography

Table 6
Comparison of the values of micropigs and normal humans measured by M-mode

†Park SW, 2000 [14].
Table 7
Comparison of the values of micropigs and normal humans measured by 2D echocardiography

†Park SW, 2000 [14].
Table 8
Comparison of the values of micropigs and normal humans measured by doppler echocardiography

†Park SW, 2000 [14].
Acknowledgments
This work was supported by Korean Rural Development Administration (BioGreen 21 Program) and Stem Cell Research Program (M10641450001-06N4145-00110), Ministry of Science & Technology, Korea.
References
1. Benzing G 3rd, Stockert J, Nave E, Tsuei YG, Kaplan S. Evaluation of canine left ventricular contractility. Cardiovasc Res. 1974. 8:313–322.

2. Bhatti FN, Schmoeckel M, Zaidi A, Cozzi E, Chavez G, Goddard M, Dunning JJ, Wallwork J, White DJ. Three-month survival of HDAFF transgenic pig hearts transplanted into primates. Transplant Proc. 1999. 31:958.

3. Buchanan JW, Bucheler J. Vertebral scale system to measure canine heart size in radiographs. J Am Vet Med Assoc. 1995. 206:194–199.
4. Crick SJ, Sheppard MN, Ho SY, Gebstein L, Anderson RH. Anatomy of the pig heart: Comparisons with normal human cardiac structure. J Anat. 1998. 193:105–119.

5. Ettinger SJ. Textbook of Veterinary Internal Medicine. 1989. 3rd ed. Philadelphia: Saunders;923–938.
6. Evans RW, Orians CE, Ascher NL. The potential supply of organ donors. An assessment of the efficacy of organ procurement efforts in the United States. JAMA. 1992. 267:239–246.

7. Gwathmey JK, Nakao S, Come PC. Echocardiographic assessment of cardiac chamber size and functional performance in swine. Am J Vet Res. 1989. 50:192–197.
8. Hardy MA. Xenograft 25. 1989. Amsterdam: Elsevier;125.
9. Korte FS, Mokelke EA, Sturek M, McDonald KS. Exercise improves impaired ventricular function and alterations of cardiac myofibrillar proteins in diabetic dyslipidemic pigs. J Appl Physiol. 2005. 98:461–467.

10. Kaczmarek I, Feindt P, Boeken U, Guerler S, Gams E. Effects of direct mechanical ventricular assistance on regional myocardial function in an animal model of acute heart failure. Artif Organs. 2003. 27:261–266.

11. Lessick J, Hayam G, Zaretsky A, Reisner SA, Schwartz Y, Ben-Heim SA. Evaluation of inotropic changes in ventricular function by NOGA maping: comparison with echocardiography. J Appl Physiol. 2002. 93:418–426.

12. Lundeen G, Manohar M, Parks C. Systemic distribution of blood flow in swine while awake or during 1.0 and 1.5 MAC isoflurane anesthesia with or without 50% nitrous oxide. Anesth Analg. 1983. 62:499–512.
13. Park SH, Kim DY, Park BK, Yoo HS, Youn HJ, Han HJ. Comparative analysis of various blood chemical values between Miniature pigs and conventional pigs. Lab Anim Res. 2006. 22:19–23.
14. Park SW. Multicenter trial for estimation of normal values of echocardiographic indices in Korea. Korean Circ J. 2000. 30:373–382.

15. Pipers FS, Muir WW, Hamlin RL. Echocardiography in swine. Am J Vet Res. 1978. 39:707–710.
16. Snedecor GW, Cochran WG. Statistical Methods. 1967. 7th ed. Edinburgh: Oliver & Boyd;156–236.
17. Stankovicova T, Szilard M, De Scheeder I, Sipido KR. M cells and transmural heterogeneity of action potential configuration in myocytes from the left ventricular wall of the pig heart. Cardiovasc Res. 2000. 45:952–960.

18. Strotman JM, Janerot-Sjoberg B, Kimme P, Frohlich B, Voigt JU, Schreckenberger AB, Hatle L, Sutherland GR. The effet of pacing-induced heart rate variation on longitudinal and circumferential regional myocardial function after acute beta-blockade-acardiac ultrasound study. Eur J Echocardiogr. 2000. 1:184–195.
19. Vogel M, Schmidt MR, Kristiansen SB, Cheung M, White PA, Sorensen K, Redington AN. Validation of myocardial acceleration during isovolumic contraction as a novel noninvasive index of right ventricular contractility: comparison with ventricular pressure-volume relations in an animal model. Circulation. 2002. 105:1693–1699.

20. Weidemann F, Jamal F, Sutherland GR, Claus P, Kowalski M, Hatle L, De Scheerder I, Bijnens B, Rademakers FE. Myocardial function defined by strain rate and strain during alterations in inotropic states and heart rate. Am J Physiol Heart Circ Physiol. 2002. 283:H792–H799.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download